Rutherford’s Gold Foil Experiment
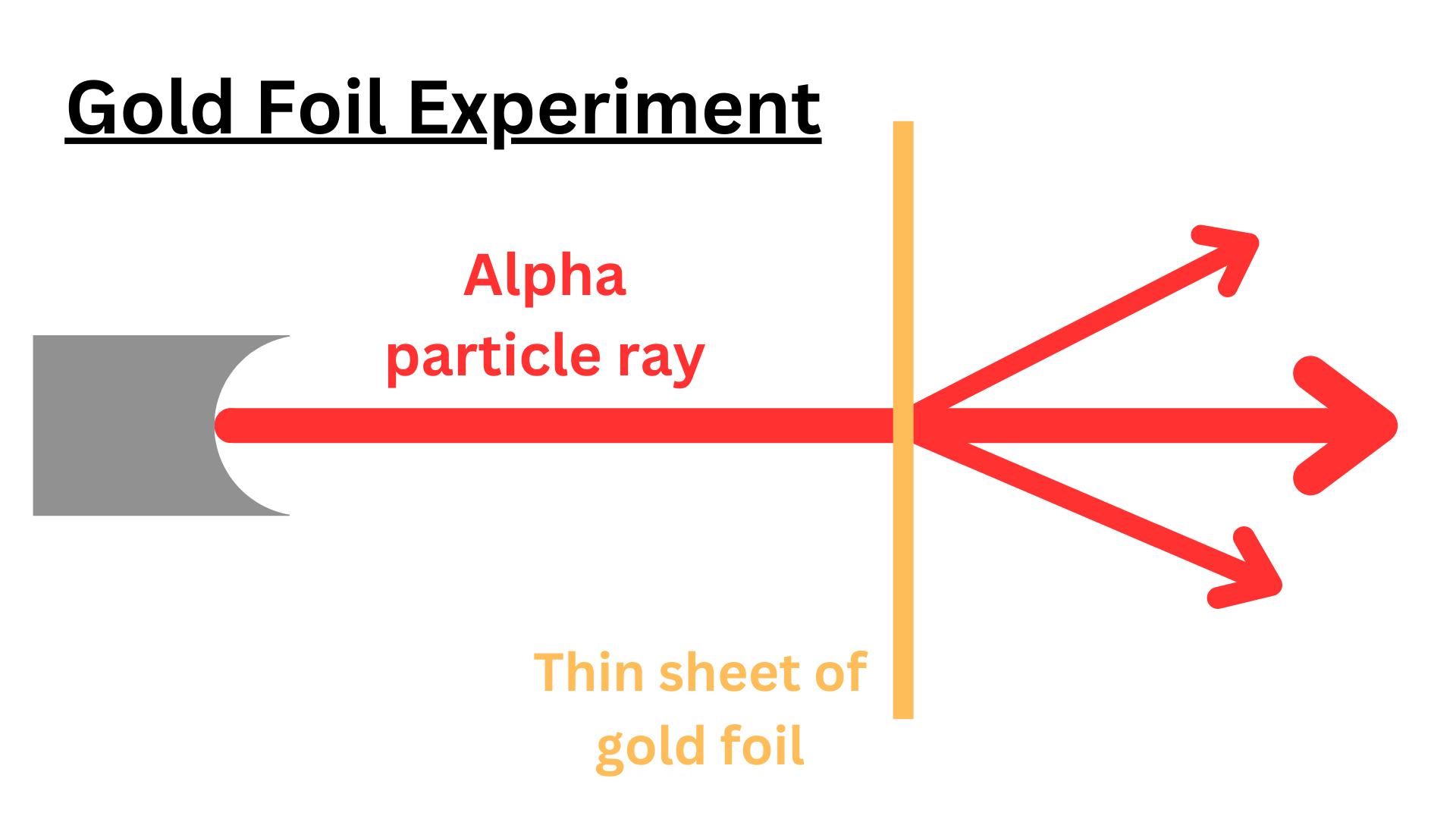
Rutherford shot alpha particles (positively charged ions) at a thin sheet of gold foil. He did this to support J.J. Thompson’s Plum Pudding Model. However, he ended up disproving it.
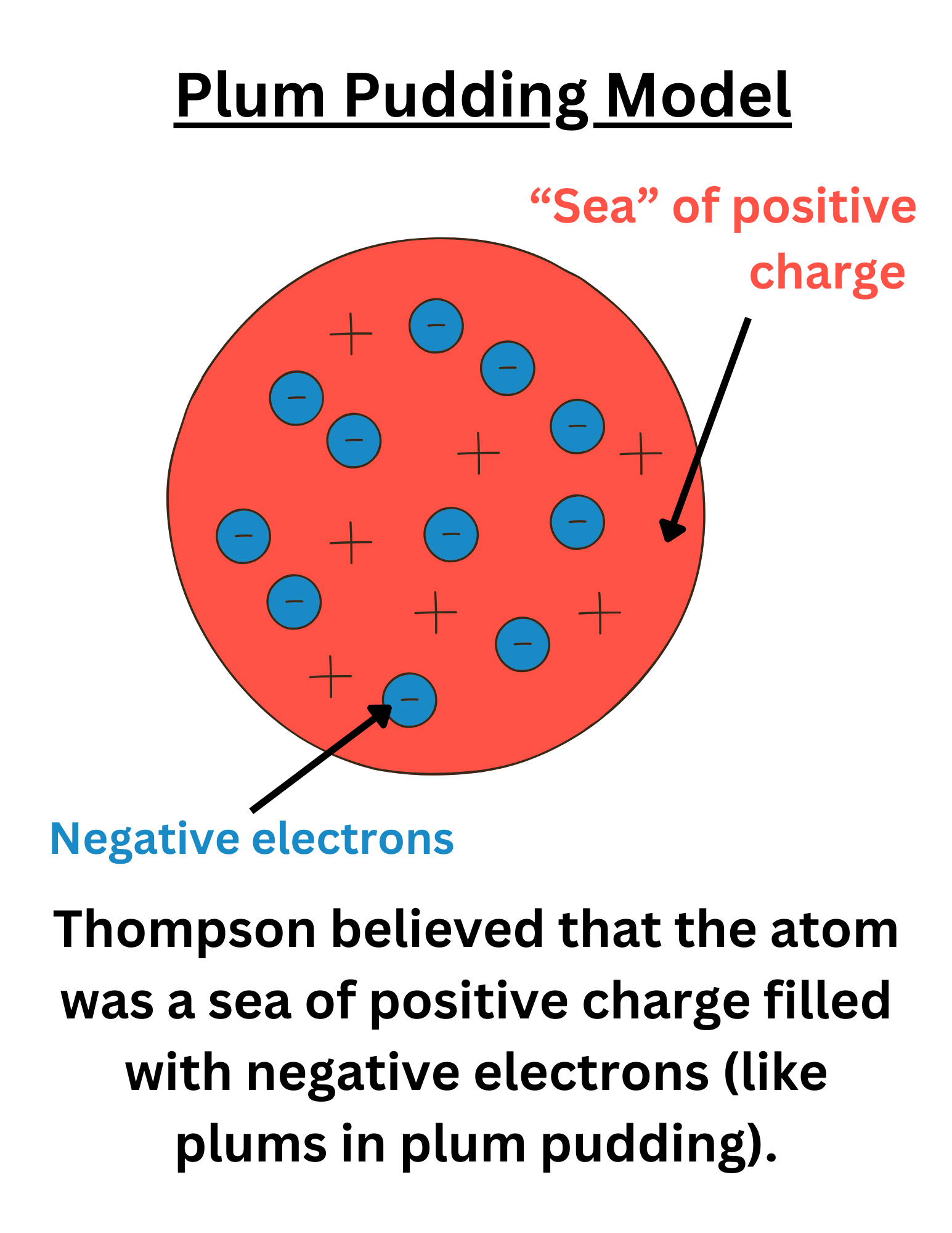
Note: the Plum Pudding Model is incorrect, as shown by Rutherford.
Conclusions
From his gold foil experiment, Rutherford made many important conclusions, majorly improving our understanding of the atom.
The atom is mostly empty space: Most of the alpha particles went through the gold sheet. This meant that most particles traveled through empty space without encountering any obstacles, indicating that atoms are mostly empty space.
There is a dense center of the atom (nucleus): Some alpha particles bounced back. This could only happen if the atom had a dense center that the alpha particle directly collided with. Because an alpha particle is much smaller than the center of a gold atom, in a direct collision, the alpha particle would bounce back. Rutherford called this dense center the nucleus.
The nucleus has a positive charge: Many alpha particles deflected at large angles. The nucleus was repelling them, so the nucleus must have the same charge as the alpha particles (same charges repel each other). Alpha particles are positively charged, so the nucleus is too.

This also showed that JJ Thompson’s Plum Pudding Model is incorrect.
Limitations
– Did not include the neutron in his model
– Could not explain how electrons were arranged
– Could not explain the stability of the atom
