These are the “principles of electron configuration,” and they are very important to understand. You should be able to identify orbital configurations that violate them.
Aufbau Principle
“Electrons fill lower energy levels before filling out higher energy levels.”
This has been discussed before, and it is the basis for electron configuration.

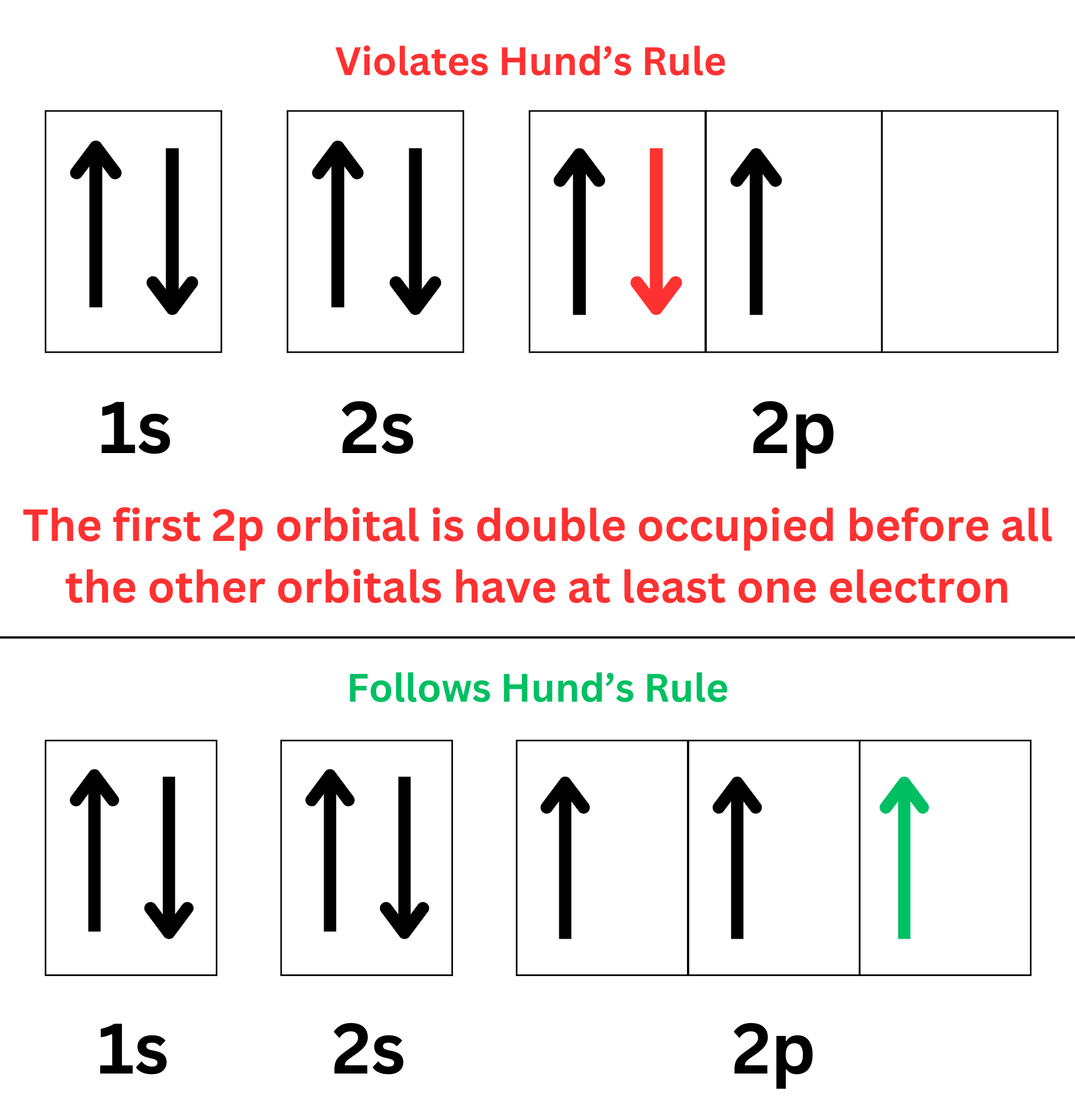
Hund’s Rule
“Electrons first fill out empty orbitals within a subshell before double occupying.”
You learned this in the “Orbital Diagram” section. In a subshell, make sure each box is filled with one electron before double-occupying a box.
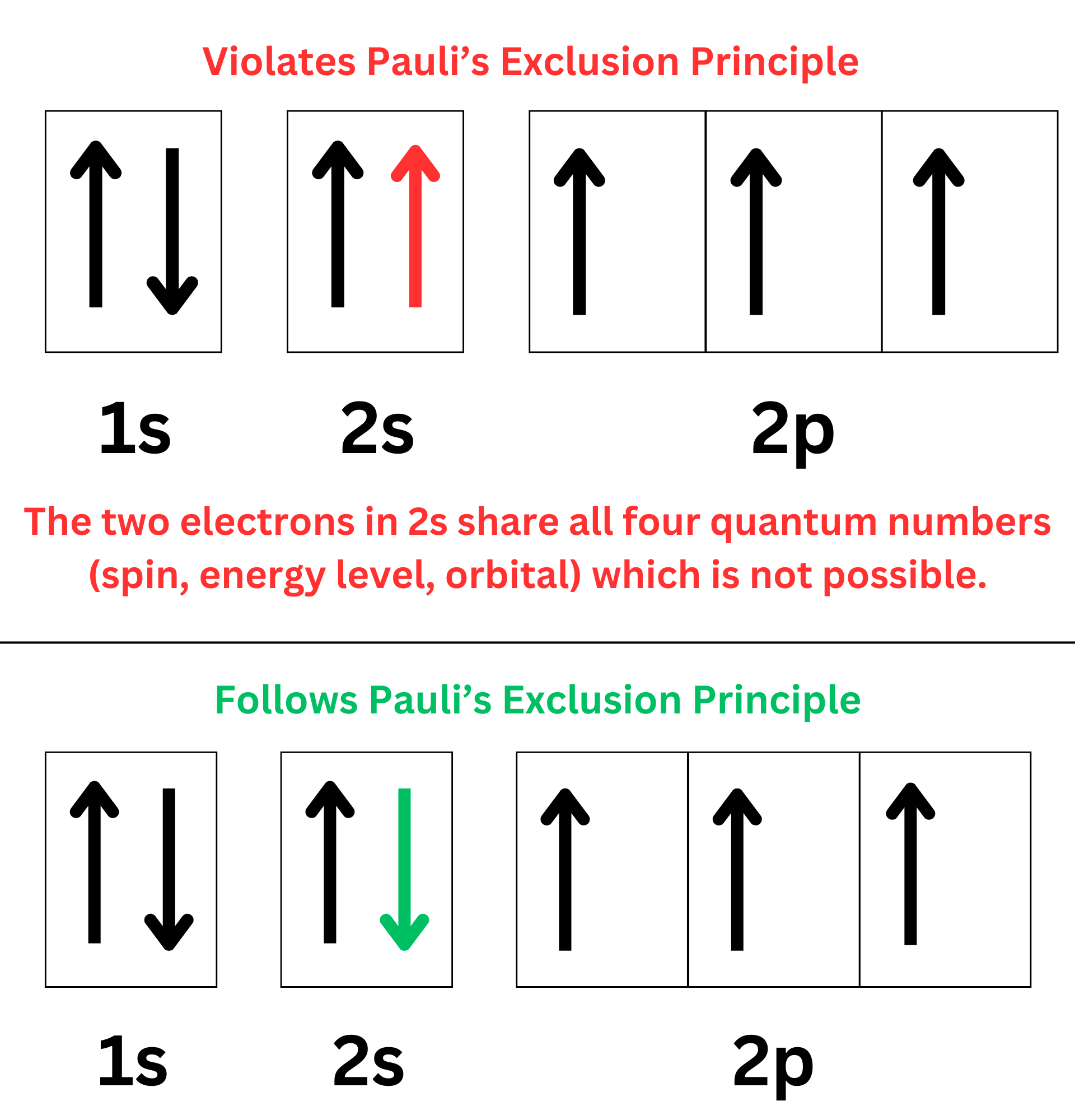
Pauli’s Exclusion Principle
“Each electron in an atom has a unique set of quantum numbers.”
This was important for quantum numbers in the “Atomic Structure” unit.