In chemistry, polarity refers to the uneven distribution of charge. We can look at the polarity of individual bonds and the polarity of molecules as a whole.
For many diagrams in this section, we do not include the lone pairs on the outer atoms because they are not important in understanding this concept.
Polarity of Bonds
In covalent bonds, electrons are shared between the two bonded atoms. In other words, the electrons orbit both atoms. However, sometimes the bonded electrons will spend more time near one atom than the other. When this happens, the bond is polar.
Recall that an atom’s ability to attract bonded electrons is called electronegativity. If there is a large enough difference in electronegativity between two bonded atoms, the shared electrons will spend significantly more time near one atom (the more electronegative atom).
Here is how we classify bonds based on the ∆EN (the difference in electronegativity between the bonded atoms) ↴
0 < ∆EN ≤ 0.4 → covalent nonpolar bond
0.4 < ∆EN ≤ 1.9 → covalent polar bond
If there is an extremely large ∆EN, one atom will completely strip the other atom of its electrons, creating charged ions that form an ionic bond.
∆EN > 1.9 → ionic bond (only a metal and a nonmetal will have that large of a ∆EN)
In a polar covalent bond, charge is unevenly distributed. The more electronegative atom carries a partial negative charge. The less electronegative atom carries a partial positive charge. These terms mean exactly what they sound like. The atoms do not have an actual charge like ions in an ionic compound, but the uneven sharing of electrons causes them to be partly charged.
Here’s an example with a bond between oxygen and hydrogen ↴

Oxygen is partially negative (δ-), because it is more electronegative. Hydrogen is partially positive (δ+), because it is less electronegative.
Dipole Moment
Not only can individual covalent bonds be classified as either polar or nonpolar, but so can entire molecules. The overall polarity of a molecule, determined by the individual polarities of each bond, is called its dipole moment.
Molecules with a dipole moment have an overall uneven distribution of charge. One side of the molecule is more negative (partial negative), while the other side is more positive (partial positive).
The diagram below shows how we represent a dipole moment, using the example HF ↴

Here’s another example, but of a molecule with more than two atoms ↴
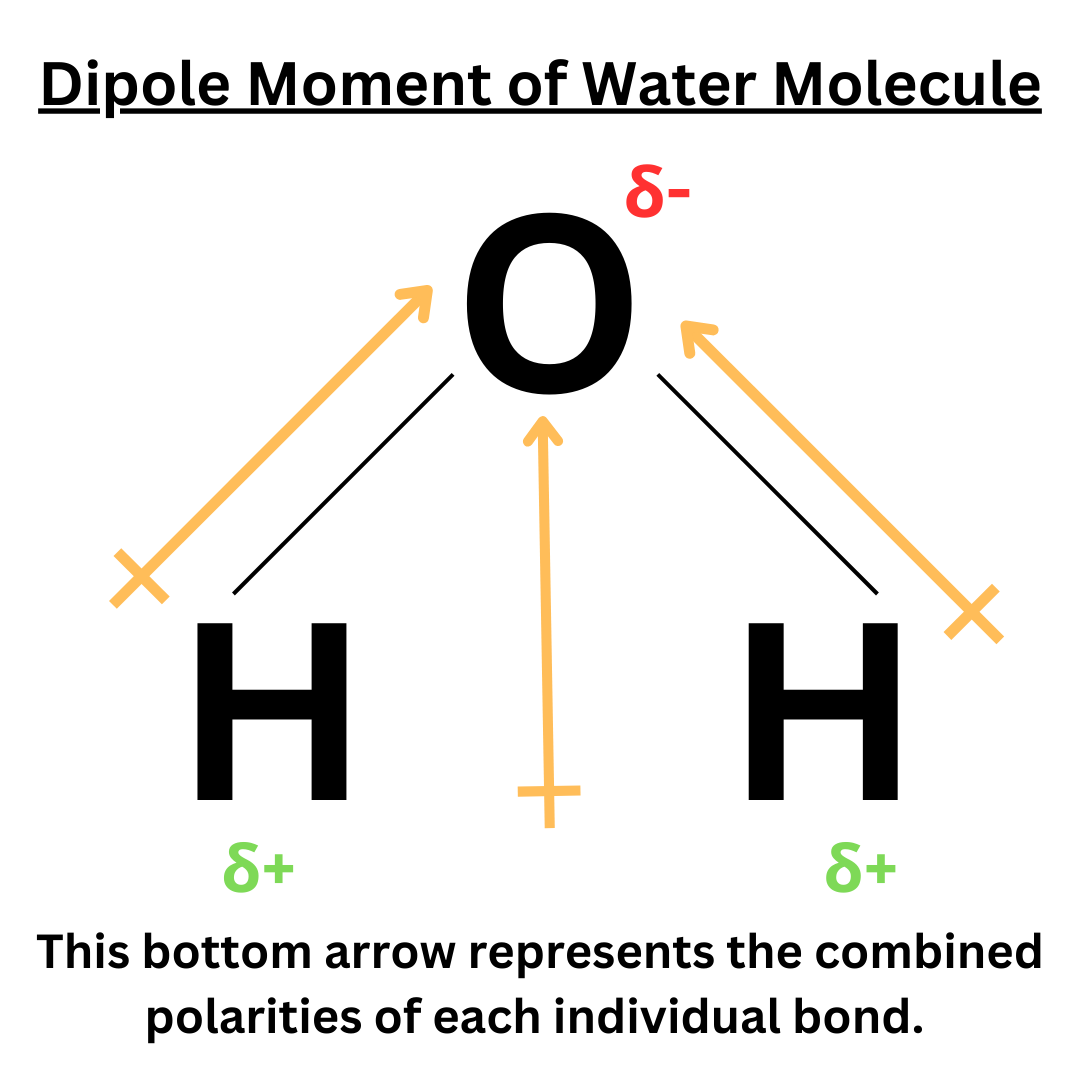
Polarity of Molecule
Some molecules do not have dipole moments. These molecules are classified as nonpolar, while molecules with an overall dipole moment are considered polar. There are three criteria to look at when determining if a molecule has a dipole moment: polarity of bonds, symmetry, and lone pairs on the central atom.
Polarity of Bonds: Of course, if the individual bonds in a molecule are all nonpolar, the overall molecule cannot be polar. However, it is very rare that you will encounter nonpolar covalent bonds, so it is safe for you to assume that individual bonds are polar. The only common example of a nonpolar covalent bond is a carbon-hydrogen bond.
Symmetry: Even though molecules may contain individual bonds that are polar, if the molecule is symmetrical, it does not have a dipole moment and is nonpolar. The polarities of individual bonds are equal but in opposite directions, canceling each other out. So overall, the molecule has an even distribution of charge.
Think about it in this way: in a game of tug of war, if the opposite sides are pulling with the same force, the rope won’t move.

The carbon-chlorine bonds in CCl₄ are all polar. However, as shown in the diagram above, these polarities cancel each other out, rendering the overall molecule nonpolar.
When we say symmetrical, we mean that you can draw at least one line of symmetry through the molecule (it does not have to be symmetrical in all ways). This will become clearer with the examples below ↴
Example #1

Example #2

Example #3

Lone Pairs (on the central atom): Most of the time, the presence of lone pairs on the central atom indicate that a molecule is polar. Remember that polarity refers to an uneven distribution of charge. Lone pair electrons are negatively charged, so their existence on the central atom affects the distribution of charge, making an atom polar.
Furthermore, they affect the shape of the molecule. Remember that electrons repel each other. When the central atom of a molecule contains a lone pair, the outer atoms are pushed away due to electron-electron repulsion. The molecule’s structure is thus altered, causing it to be asymmetrical and polar. You will learn about this more in the “Chemical Bonding” unit.
Water is a great example ↴

