Delocalized Electrons
Delocalized electrons are not confined to a single atom or covalent bond.
In a normal covalent bond, the shared electrons will orbit and spend time near two atoms. Delocalized electrons may orbit three or more atoms. Essentially, they are distributed between (can travel between) more than two atoms.
Resonant Structures
The structure of some molecules cannot be described through a single Lewis Dot diagram. This issue arises with molecules that can be drawn in multiple ways while maintaining stability. For example, look at these two Lewis Dots of nitrite (NO₂⁻) ↴
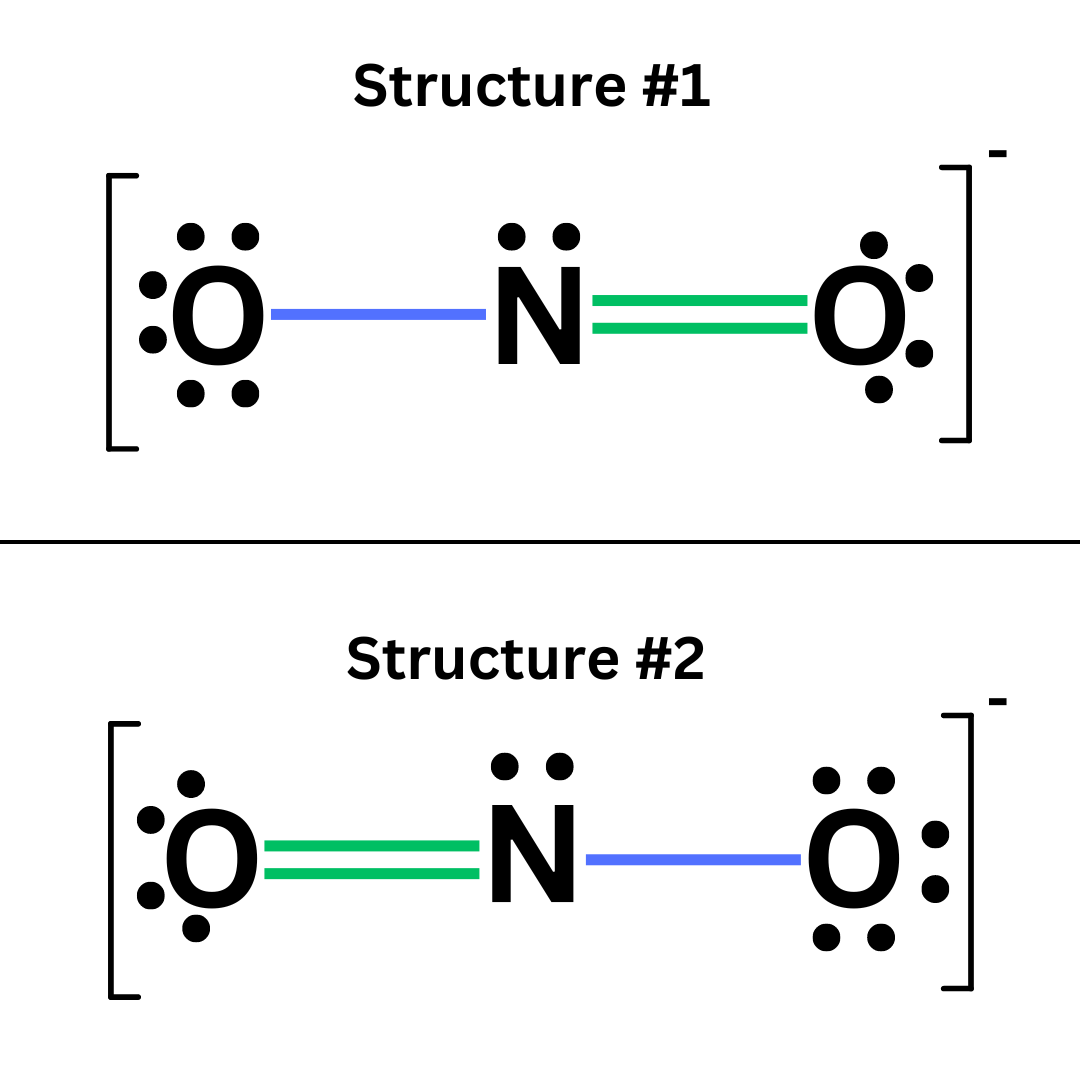
These structures are essentially the same; they are equally stable. We call these resonant structures.
Resonant structures are two or more equally stable structures of a molecule that collectively describe the molecule’s overall structure. When a molecule has a double or triple bond that can be moved between multiple atoms, it has resonant structures. This occurs often for polyatomic ions.
Individually, each resonant structure does not describe the true structure of a molecule. The actual molecule is a combination of its resonance structures. In other words, the true structure of the molecule is the “average” of its resonant structures.
Ex. A bond is depicted as a double bond in one resonant structure and a single bond in another. It is actually between a single and double bond (like a “1.5 bond”). Thus, the bond length is shorter than a single bond but longer than a double bond.
Here’s how we represent resonant resonance structures through Lewis Dot diagrams ↴
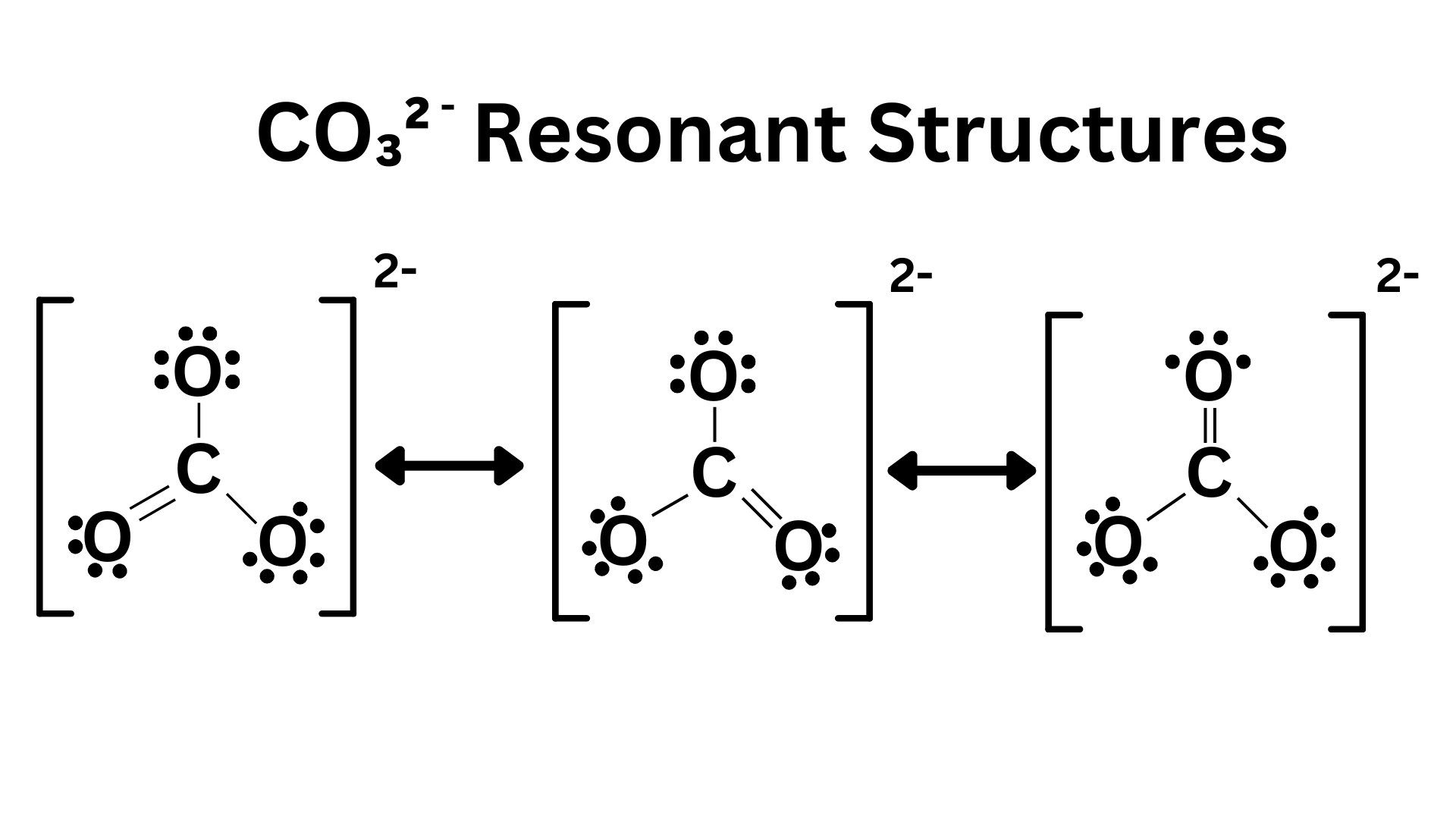
The double sided arrows between the different resonant structures indicate that the molecule is a combination/mixture of all three.
