Electromagnetic radiation is a type of energy that travels in the form of waves. Visible light is a type of electromagnetic radiation; however, there are many other types.
We call individual packets of electromagnetic radiation photons.
Electromagnetic Spectrum

The above image shows all of the types of electromagnetic radiation. As you can see, most electromagnetic radiation is not visible to the human eye (pun intended).
Wavelength, Frequency, and Energy
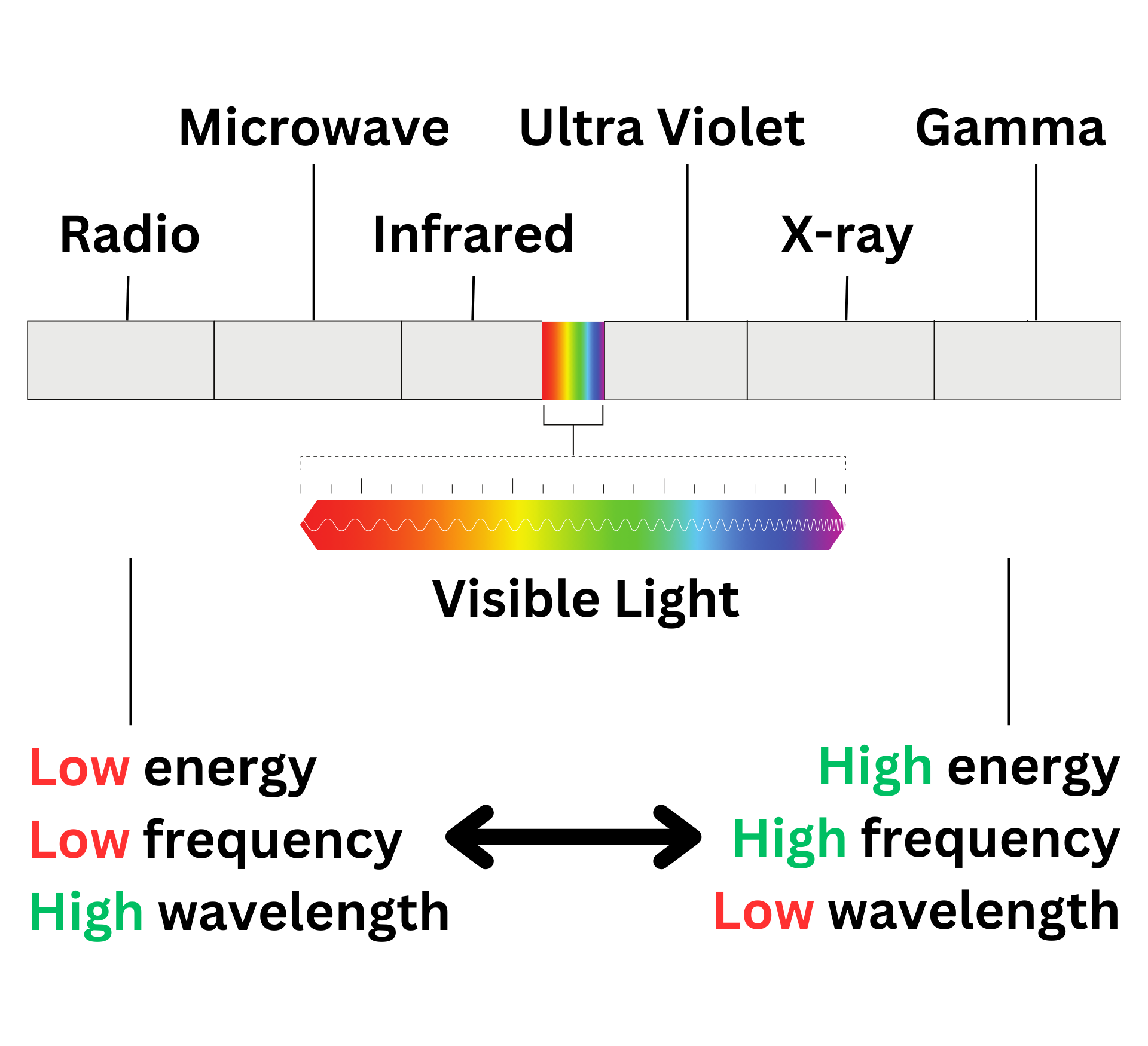
As you move towards gamma radiation, the energy and frequency of the wave increases, while the wavelength decreases. Moving towards radio waves does the opposite.
Based on this, we know that wavelength and frequency are inversely proportional, while frequency and energy are directly proportional. Two crucial equations were made using this idea ↴
C = λf
– C is the speed of light (3 x 10⁸ m/s). All electromagnetic radiation travels at the speed of light.
– λ is the wavelength of the photon (in meters)
– f is the frequency of the photon (in Hz or 1/s)
– This also displays how λ and f are inversely proportional
– Note that because the speed of light is measured in m/s, when using this equation, λ must be in meters and f must be in Hertz
E = hf
– E represents the energy of the photon
– h is Planck’s constant (6.626 x 10⁻³⁴ Js)
– f is frequency of the photon (in Hz or 1/s)
– This also shows how energy and frequency are directly proportional
– Note that because Planck’s constant is Joules * seconds, E will be in Joules
Know these two equations very well. You could be given wavelength, energy, or frequency, and need to know how to convert between the three.
To convert between energy and wavelength, you will need to use both equations, using frequency as a bridge between the two. For example, if you have the energy of a photon and want its wavelength, you must first find its frequency using E = hf. Then you can find its wavelength using C = λf.
Line Spectra
Sources of light (typically stars) emit light, forming the continuous spectrum. A gas then absorbs and emits some of this light, forming the absorption and emission spectra.

Continuous Spectrum: Includes all visible light. That is why it is “continuous.” Stars emit continuous spectra because they emit all frequencies of light.
Emission Spectrum: Strips of light on a dark background. The light strips show light that was absorbed, and then later emitted by the gas.
Absorption Spectrum: Strips of dark on a colorful background. The dark strips indicate colors that were absorbed and then later emitted. The colorful area shows the light that was not absorbed and passed directly through the gas.
If you combine the emission and absorption spectra, you get the continuous spectrum.
Emission and absorption spectra are different for each atom. This is because different atoms absorb different frequencies of light. Scientists can therefore use these spectra to identify an atom.
