Electron affinity measures the energy released when an atom gains an electron. You can think of it as how much the atom “wants” to gain an electron (but don’t write that on your test). Removing an electron is endothermic (the atom must absorb energy), but adding an electron is exothermic (the atom releases energy).
X + e⁻ → X⁻ + Energy
(chemical reaction for the electron affinity of element “X”. Notice how energy is a product, meaning that it is released.)
When we refer to a “greater” electron affinity, we mean a more negative electron affinity value. Energy release is represented by a negative value, so a more negative electron affinity means that more energy is released.
Electron affinity decreases as you move down a group. Moving down a group, there are more principal energy levels. The valence electrons are further from the nucleus, and experience a stronger shielding effect. Consequently, the added electron is less attracted to the nucleus. The nucleus will do less work (release less energy) to gain that electron because there is a weaker attraction.
The only exception to this is the second period (nitrogen, oxygen, fluorine, etc.). They have a lower electron affinity than the third period (phosphorus, sulfur, chlorine, etc.). Because of this, chlorine has the highest electron affinity (not fluorine).
Moving across a period, there is no strict/easy trend. Many sources say that it generally increases moving left to right, but this does not always hold true.
The more specific pattern is 76 45 13 28. These numbers refer to the main group numbers (7 refers to group 7A, 6 refers to group 6A, and so on). As you go from left to right across 76 45 13 28, electronegativity decreases. So group 7A has the highest electron affinity (most negative), and group 8A has the least electron affinity (most positive).
We will explain why below ↴
Why 7A and 6A have the Greatest Electron Affinity
To understand this, you must understand that atoms release energy as they become more stable.

Elements in Group 7A have the greatest electron affinity because gaining an electron completely fills their valence shell. 7A elements have 7 valence electrons, and 8 valence electrons are needed to reach stability (all atoms “want” stability), so they will do a great amount of work to reach that stability. In other words, they will release lots of energy to gain that extra electron. You could think of it as 7A elements really “wanting” the extra electron (but do not write “want” on your test).
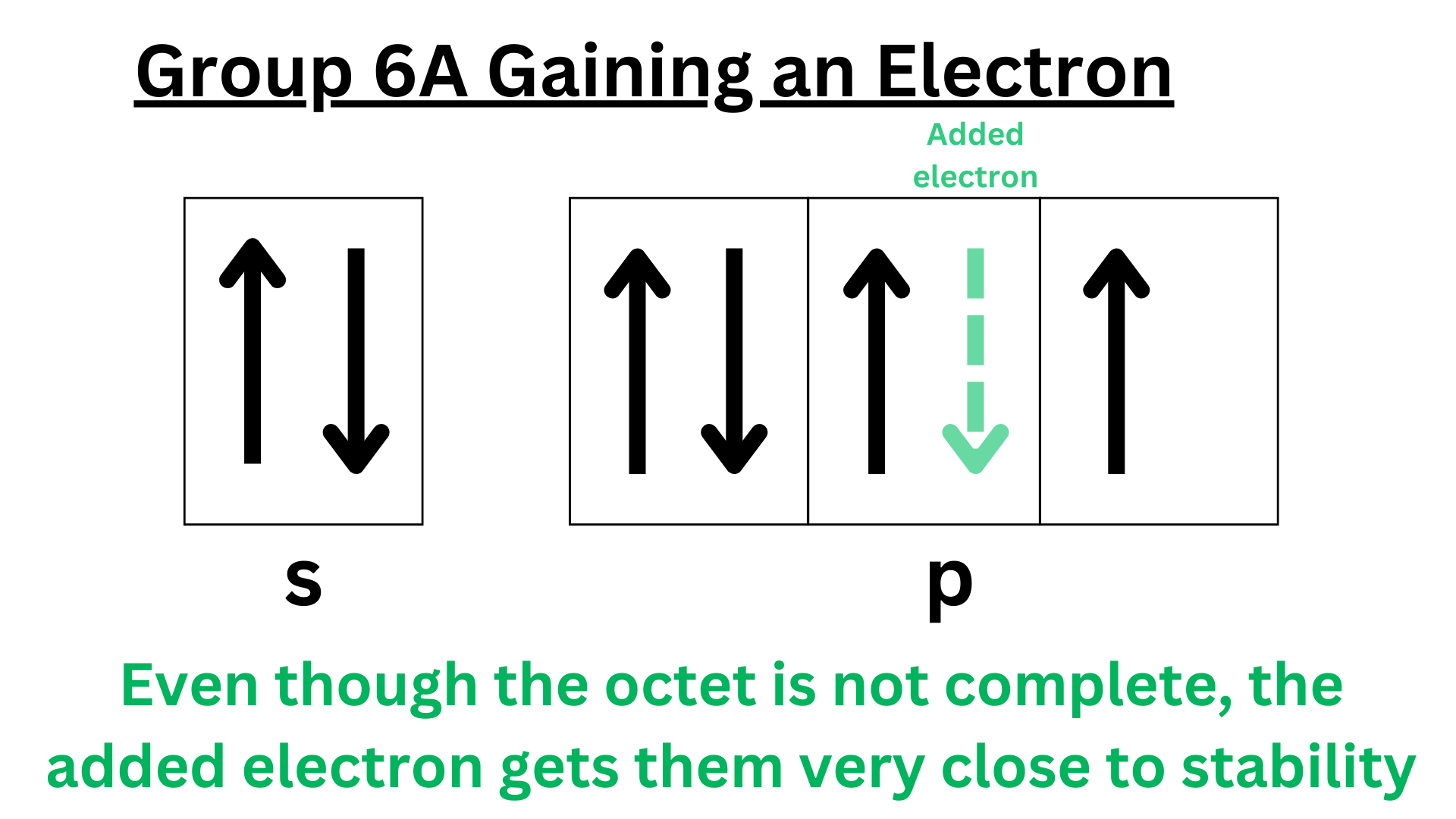
Similar occurs for group 6A. Elements in this group have 6 valence electrons. Gaining an electron puts them extremely close to having 8 valence electrons. Group 6A elements will release lots of energy to gain an electron and become more stable. However, gaining this electron does not fully complete their octet as it does for group 7A. That’s why group 7A has a higher electron affinity.
Why 2A and 8A have the Least Electron Affinity
Group 2A and 8A elements actually have a positive electron affinity. They do not release energy when gaining an electron. In fact, they will absorb energy. Here’s why ↴

Elements in group 2A have a positive electron affinity. They already have their s-subshell filled with 2 electrons, so they are pretty stable. Adding an electron makes them less stable, because now they have a partially filled p-subshell. Because the atom is becoming less stable, you must essentially “force it” to gain the electron by supplying it with energy.
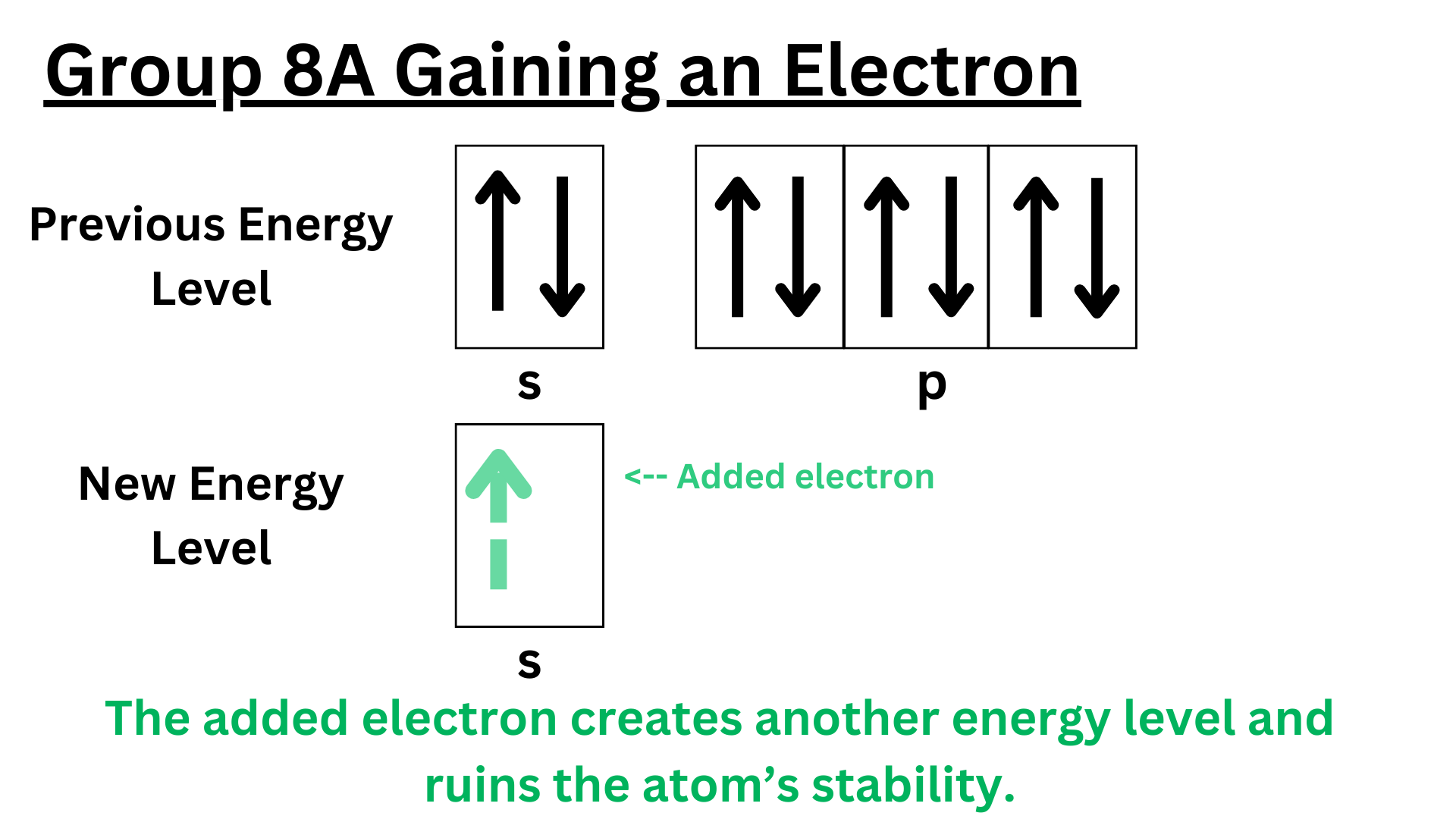
Elements in group 8A have an even more positive electron affinity then elements in group 2A. Remember that noble gasses are the most stable elements on the periodic table. They do not want to change at all. They already have a full valence shell. Gaining an extra electron would make a noble gas a lot less stable. Therefore, the atom will not release energy to gain an electron. Similar to group 2A, it will absorb energy instead.
