Electronegativity describes the ability of an atom to attract electron(s) in a bond. It is very important in later units when discussing chemical bonding.
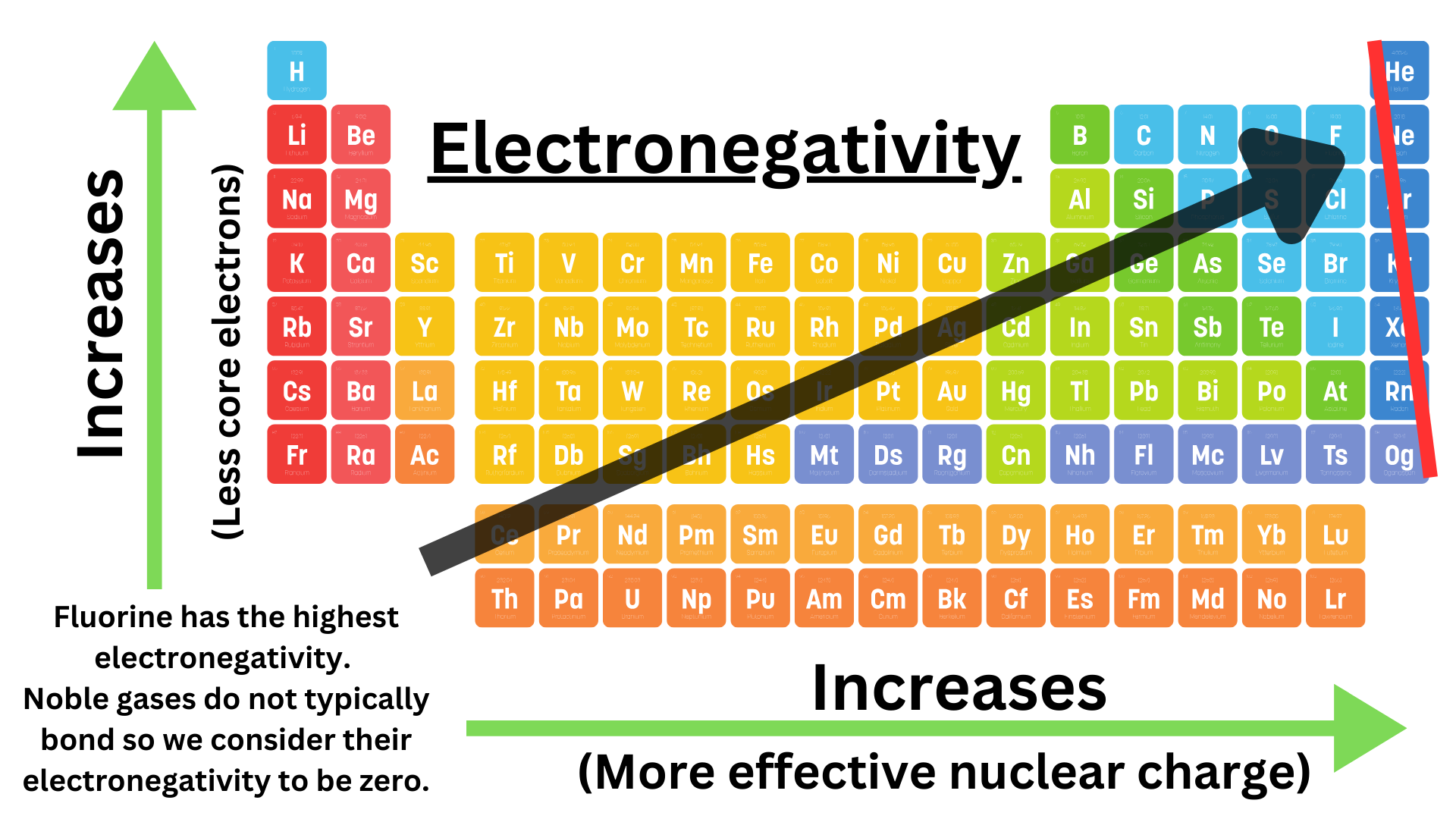
Elements are given electronegativity values from 0.7 to 4.0. Fluorine has the highest electronegativity (4.0). A high electronegativity indicates that the atom will strongly attract electrons in a bond. In a bond, the atom with the higher electronegativity will “hog” the electrons more.
Bond Types
The difference in electronegativity between two atoms can help us classify the bond between them.
If you have a covalent compound (nonmetal + nonmetal), you can classify the bond between the two atoms as either nonpolar or polar.
– A nonpolar bond is where there is small difference in electronegativity between the atoms, and so electrons are shared equally.
– A polar bond is where there is a larger difference in electronegativity. The electrons are pulled to the atom with a greater electronegativity. However this difference is not too large. Electrons are still shared, but shared unequally.
However, if you have an ionic compound (metal + nonmetal) you would simply classify the bond as an ionic bond. The nonmetal has a much higher electronegativity than the metal. This results in the nonmetal completely taking an electron from the metal.
(note ∆EN refers the difference in electronegativity between the two atoms)
If ∆EN < 0.4, it is a nonpolar covalent bond.
If 0.4 < ∆EN < 1.9, it is a polar covalent bond.
If ∆EN > 1.9, it is an ionic bond.
The Trend
Moving down a group decreases electronegativity. Moving down a group adds more energy levels. The valence electrons, which participate in bonds, are further from the nucleus. There will also be more core electrons, so the bonding valence electrons experience a greater shielding effect. The bonding electrons are less attracted to the nucleus, so electronegativity has decreased.
Moving left to right across a period increases electronegativity. As you move left to right, the amount of protons increases, while the amount of core electrons remains the same. This means that effective nuclear charge increases. Bonding electrons experience a stronger attraction to the nucleus, indicating increased electronegativity.
Note that we assign noble gasses electronegativities of zero because they do not typically form bonds.
Example Problem #1
Which atom is more electronegative?
B vs. Al
Answer
Boron is more electronegative. Boron has less principal energy levels than aluminum, so bonded electrons will be closer to boron’s nucleus. The bonded electrons therefore experience a greater attraction to boron.
Example Problem #2
Which atom is more electronegative?
Al vs. S
Answer
Sulfur is more electronegative. They have the same amount of principal energy levels, but sulfur has more protons. Hence, sulfur has a greater effective nuclear charge. This means that bonding electrons will be more attracted by sulfur.
Example Problem #3
Which atom is more electronegative?
Rb vs. Ca
Answer
Calcium is more electronegative. Calcium has less principal energy levels and a greater effective nuclear charge, so it is better able to attract bonding electrons.
