Excited State
When atoms bond, only unpaired electrons can participate in bonding. In that case, how does carbon form 4 bonds? When we look at an orbital diagram of carbon, it only has 2 unpaired electrons, so it should only be able to form 2 bonds ↴

Back in the “Atomic Structure” unit, we discussed how electrons can move up energy levels by absorbing energy from light. In order to form four bonds, carbon electrons become excited, moving to an energy level where they are unpaired ↴

Now, carbon has four unpaired electrons, allowing it to form four bonds. When electrons move up energy levels to become unpaired, the atom is in its excited state. Normally, the atom is in its ground state. When bonding, atoms are in their excited state.

Expanded Octet
In the molecule SF₆, sulfur is a central atom and has single bonds with 6 fluorine atoms. This means that the sulfur has 12 valence electrons instead of 8, which violates the octet rule ↴

However, sulfur’s highest energy level is the third energy level (n = 3), which means it can access the d orbital (3s, 3p, and 3d are all part of the 3rd energy level). In its excited state, two of sulfur’s electrons expand into those 3d orbitals ↴

As you can see, excited sulfur has 6 unpaired electrons, giving it the ability to form 6 bonds. This is why it can remain stable while having more than 8 electrons (violating the octet rule). We call this an expanded octet.
Third row and below: Only elements in the third row of the periodic table (third period) and below can have expanded octets.
This is because these elements have access to a d-orbital. When they become excited, they can shift electrons into the d-orbital, allowing them to have more than 4 unpaired electrons. Therefore, they can form more than 4 bonds, and have more than 8 valence electrons.

Sometimes in your Lewis Dot diagrams, the central atom will have an expanded octet. This is allowed if it is in the “third row or below”.
However, elements above the third row, such as nitrogen, oxygen, carbon, and fluorine, can not have an expanded octet. If any of these elements are your central atom, they cannot have an expanded octet.
Connection to Hybridization
Looking through the lens of unpaired electrons, we can better understand the idea of hybridization, sigma bonds, and pi bonds.
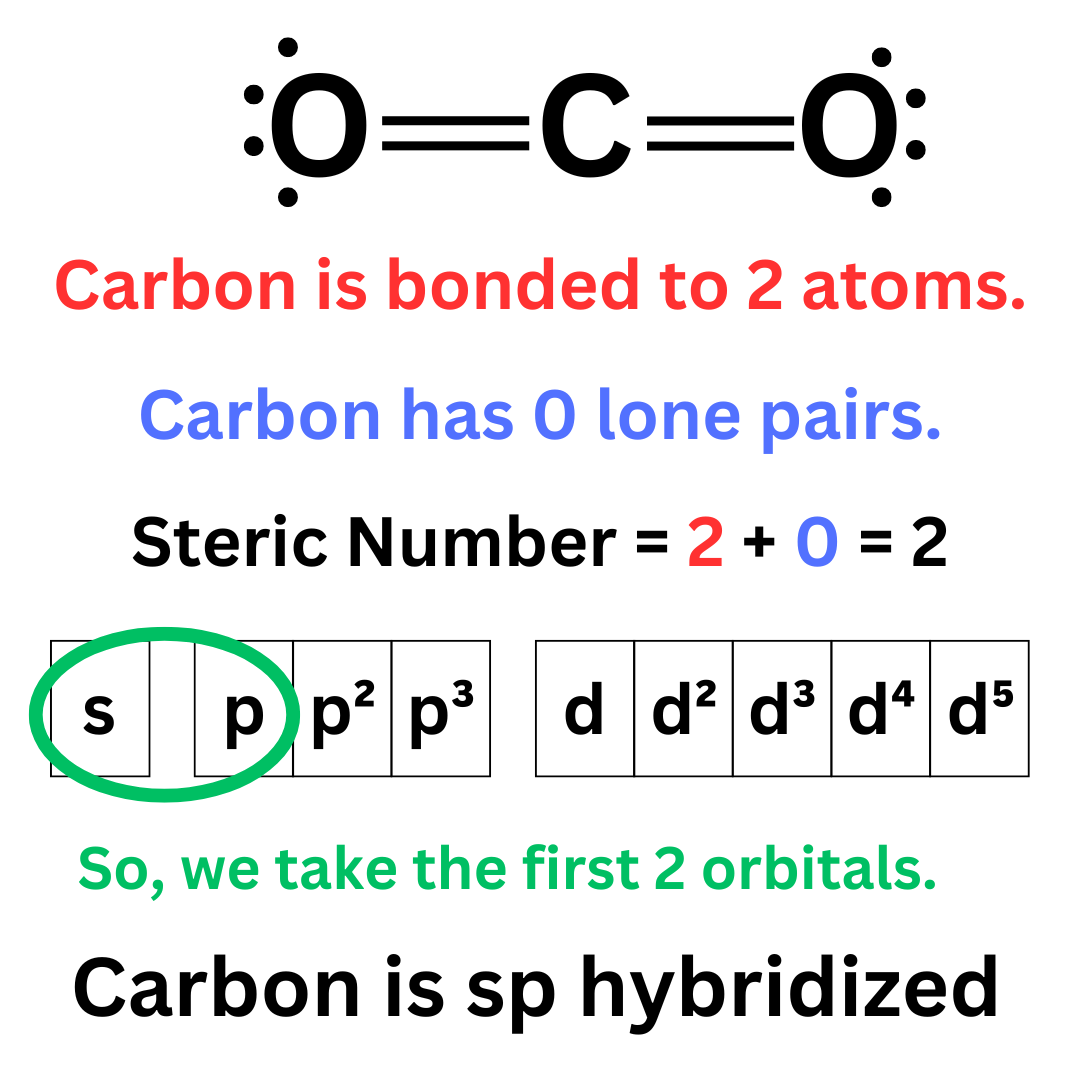
Let’s look at the CO₂ molecule. As aforementioned, carbon in its excited state will have 4 unpaired electrons ↴

In CO₂, the central carbon is sp hybridized ↴
Looking back at the orbital diagram of the excited carbon atom, we can identify which orbitals are hybridized (involved in the hybrid orbital).

The unpaired electrons in hybridized orbitals will form sigma bonds. However, we still have 2 unpaired electrons in orbitals that are unhybridized.

These unhybridized orbitals form pi bonds. They form the second bond in the carbon-oxygen double bonds.
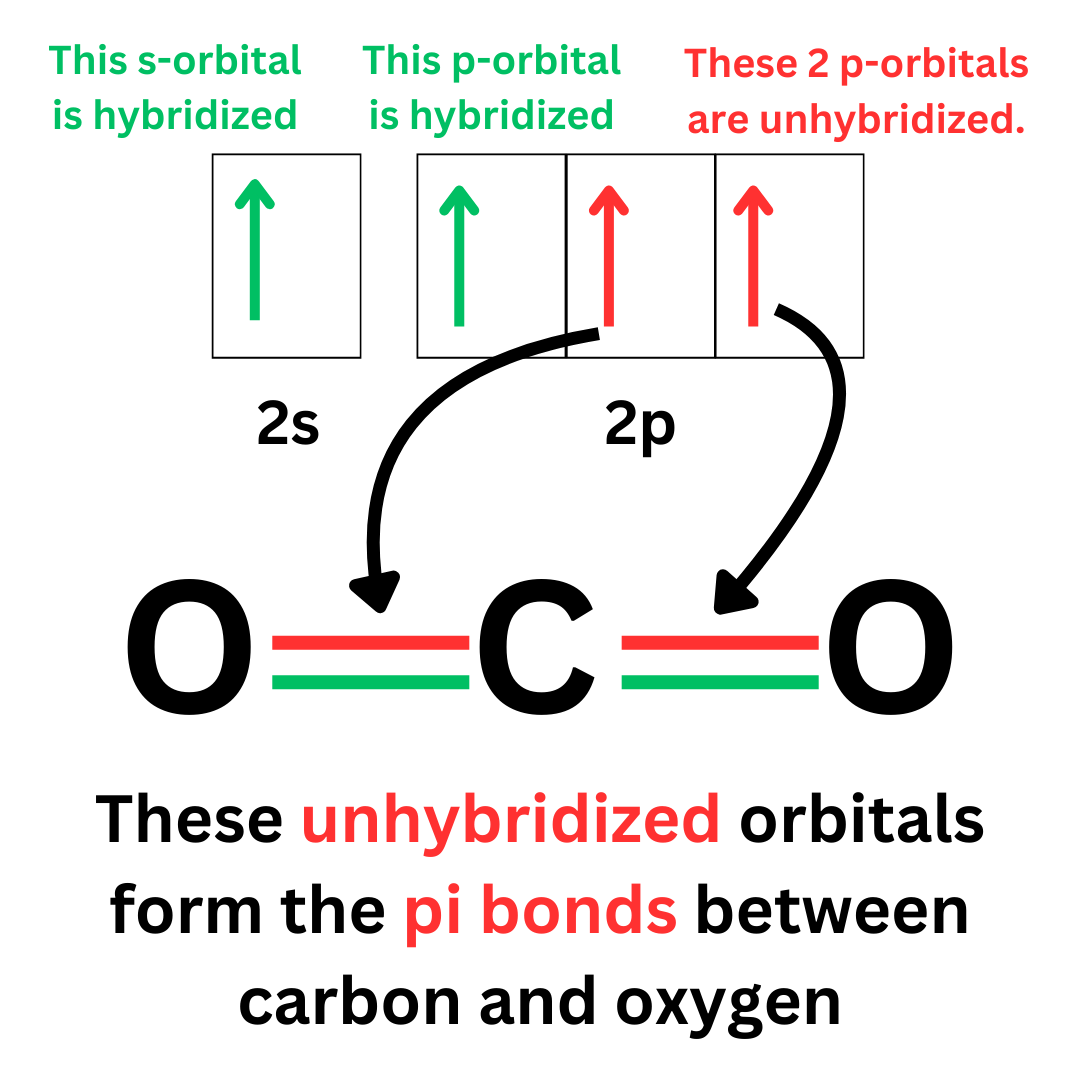
Hybridized orbitals form sigma bonds. Unhybridized orbitals form pi bonds.
