VSEPR
VSEPR, or the Valence Shell Electron Pair Repulsion theory, allows us to determine the shape of a molecule. It states that atoms will arrange themselves in a way that minimizes repulsions between valence electrons.
As we’ve stated many times before, electrons repel each other because they have the same charge (negative). In a molecule, the lone pair electrons on atoms repel each other, pushing the atoms as far apart from each other as possible. In other words, electron-electron repulsion causes atoms in a molecule to become as spread out as possible.

As you can see, the electrons repel each other, causing the atoms to be spread out in a way that minimizes electron-electron repulsion.
Molecular Domain
Using VSEPR theory, we can classify the geometries of molecules based on the bonds and lone pairs on the central atom. Note that our focus is on the central atom. We do not care about lone pairs on the outer atoms when determining a molecule’s geometry.
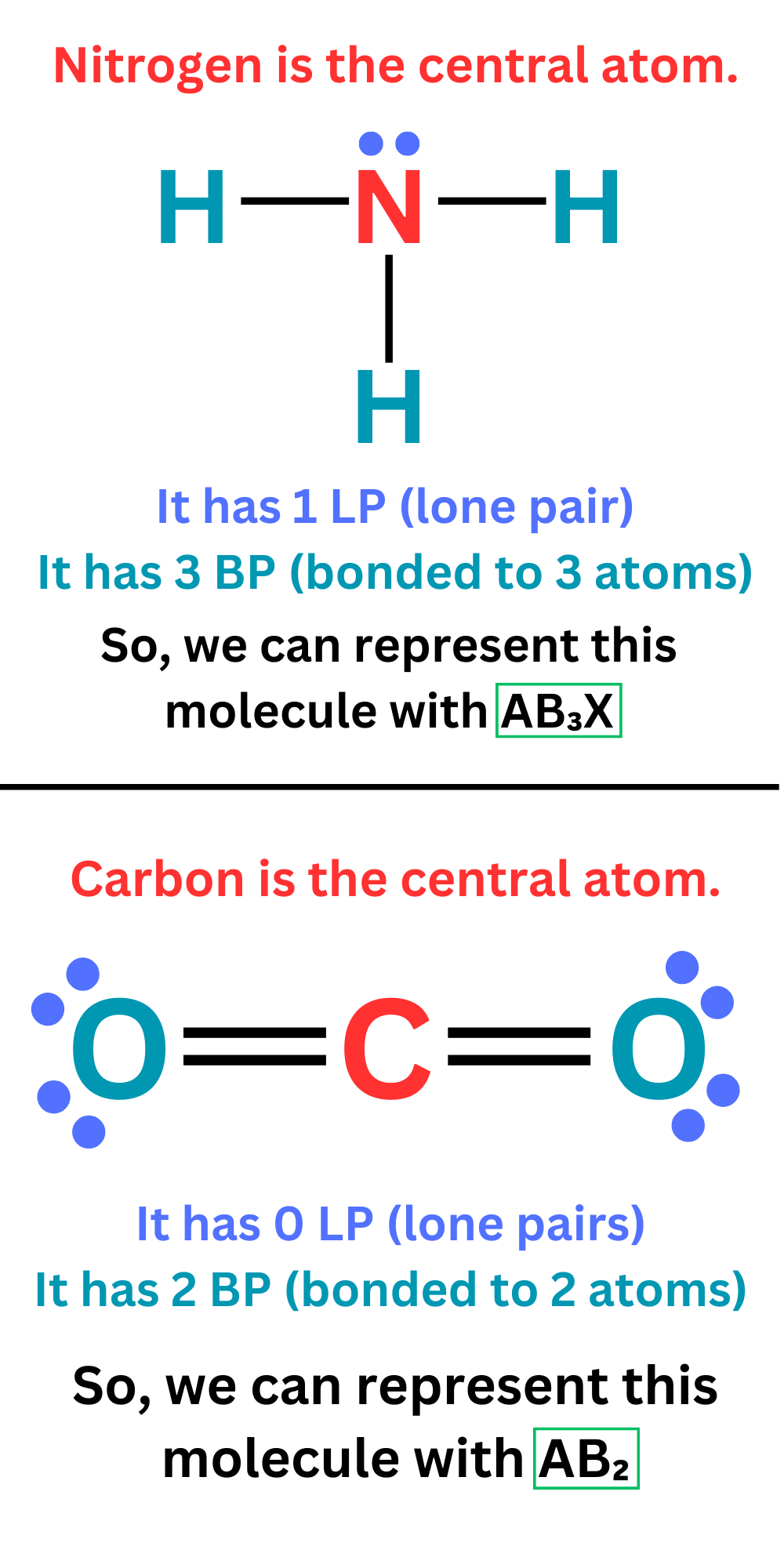
We use the notation ABₘXₙ, where
– A represents the central atom
– B stands for the outer atoms, the subscript m is the number of atoms bonded to the central atom- X stands for lone pairs, the subscript n is the number of lone pairs on the central atom
Ex. AB₂X would represent a molecule with two atoms bonded to the central atom, and one lone pair on the central atom.
Ex. AB₃X₂ would represent a molecule with three atoms bonded to the central atom, and two lone pairs on the central atom.
Ex. AB₄ would represent a molecule with four atoms bonded to the central atom, and no lone pairs on the central atom.
We can write the ABₘXₙ of a molecule by looking at its Lewis Dot structure.
Then, based on the ABₘXₙ notation, we can name the molecular geometry of the molecule. We can also determine the bond angle of the molecule (the angle formed between two bonds in a molecule).
Lewis Dots are 2D, while actual molecules are 3D. Recognize that bond angles describe the angle between bonds in a three-dimensional molecule, not necessarily the angles depicted in a two-dimensional Lewis Dot. So, you may look at a Lewis Dot Structure and think that it doesn’t accurately depict the molecule’s bond angle. You would be right: Lewis Dots are 2D while real molecules are 3D.
You are going to have to memorize the names of these geometries. Here is a chart of the molecular geometries and their corresponding bond angles ↴

Electron Domain
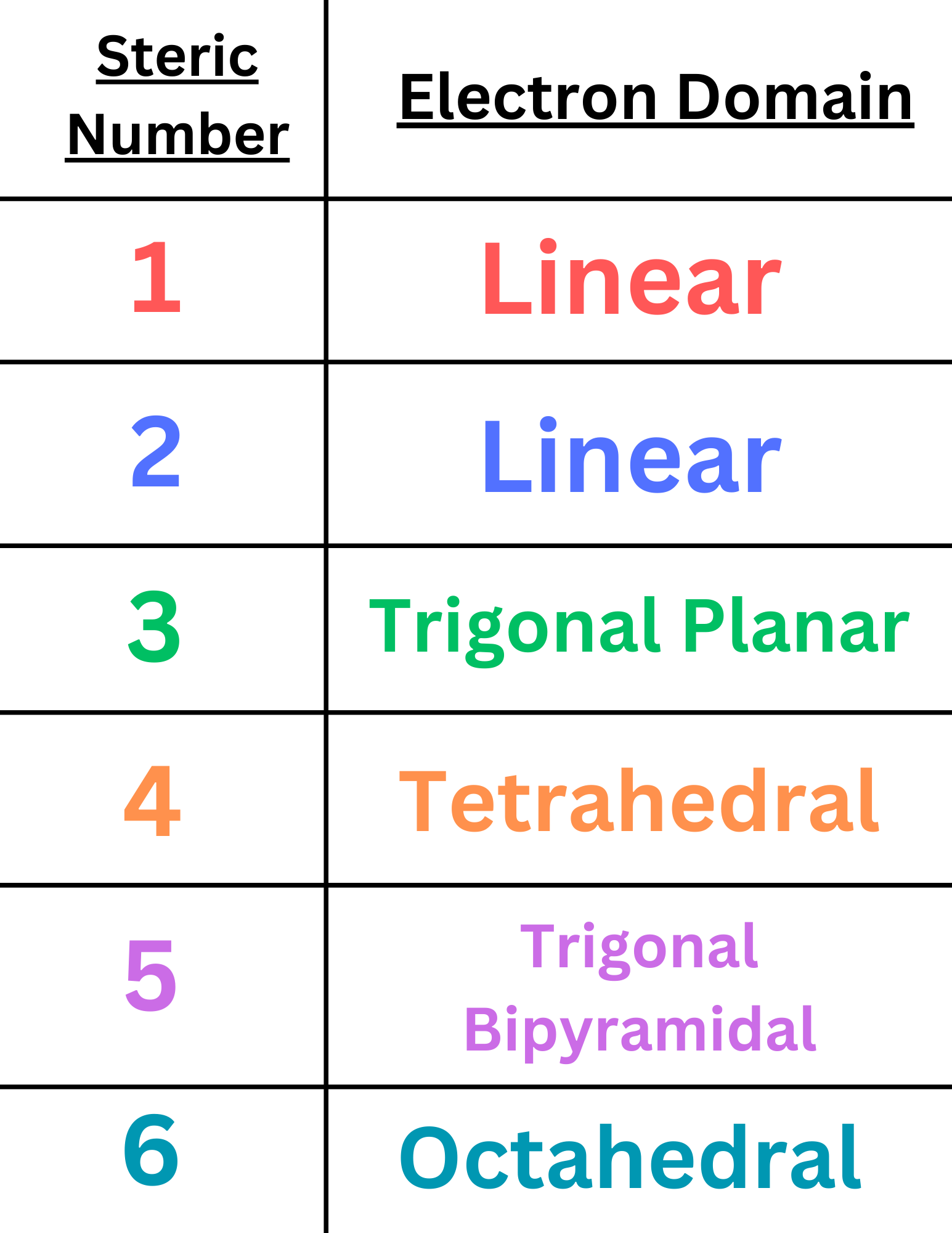
Electron domain is slightly different from the molecular domain. When determining molecular domain, we distinguish between the number of lone pairs and bonded atoms. However, for electron domain, we do not make this distinction. We determine electron domain based on the steric number of a molecule.
Steric Number = (Number of atoms bonded to the central atom) + (Number of lone pairs on the central atom)
As you can see, in calculating steric numbers, we treat bonded atoms and lone pairs as the same. For example, a molecule with a tetrahedral molecular domain (AB₄) has a steric number of 4. Yet, a molecule with a trigonal pyramidal molecular domain (AB₃X) also has a steric number of 4.
Based on the steric number, we can determine the electron domain ↴