Hess’ Law states that if a reaction occurs in multiple steps, the ∆H for the total reaction is the sum of the ∆H’s of the individual steps.
∆H(total) = ∆H(step 1) + ∆H(step 2) + ∆H(step 3)… and so on
Also, the ∆H for the reverse reaction is equal to -∆H for the forward reaction.
Ex. O₆ + C₆H₁₂O₆ → 6CO₂ + 6H₂O has a ∆H(comb) of -2840 kJ/mol. If we were to flip the reaction, it would negate the ∆H.
So 6CO₂ + 6H₂O → O₆ + C₆H₁₂O₆ has ∆Hᵣₓₙ = 2840 kJ/mol.
If you multiply the coefficients of a reaction by n, then the ∆Hᵣₓₙ multiplies by a factor of n.
Ex. O₆ + C₆H₁₂O₆ → 6CO₂ + 6H₂O has a ∆H(comb) of -2840 kJ/mol. If we were to double the coefficients ↴
2O₆ + 2C₆H₁₂O₆ → 12CO₂ + 12H₂O
Then the ∆H(comb) = -5680 kJ/mol, which is double the original enthalpy.
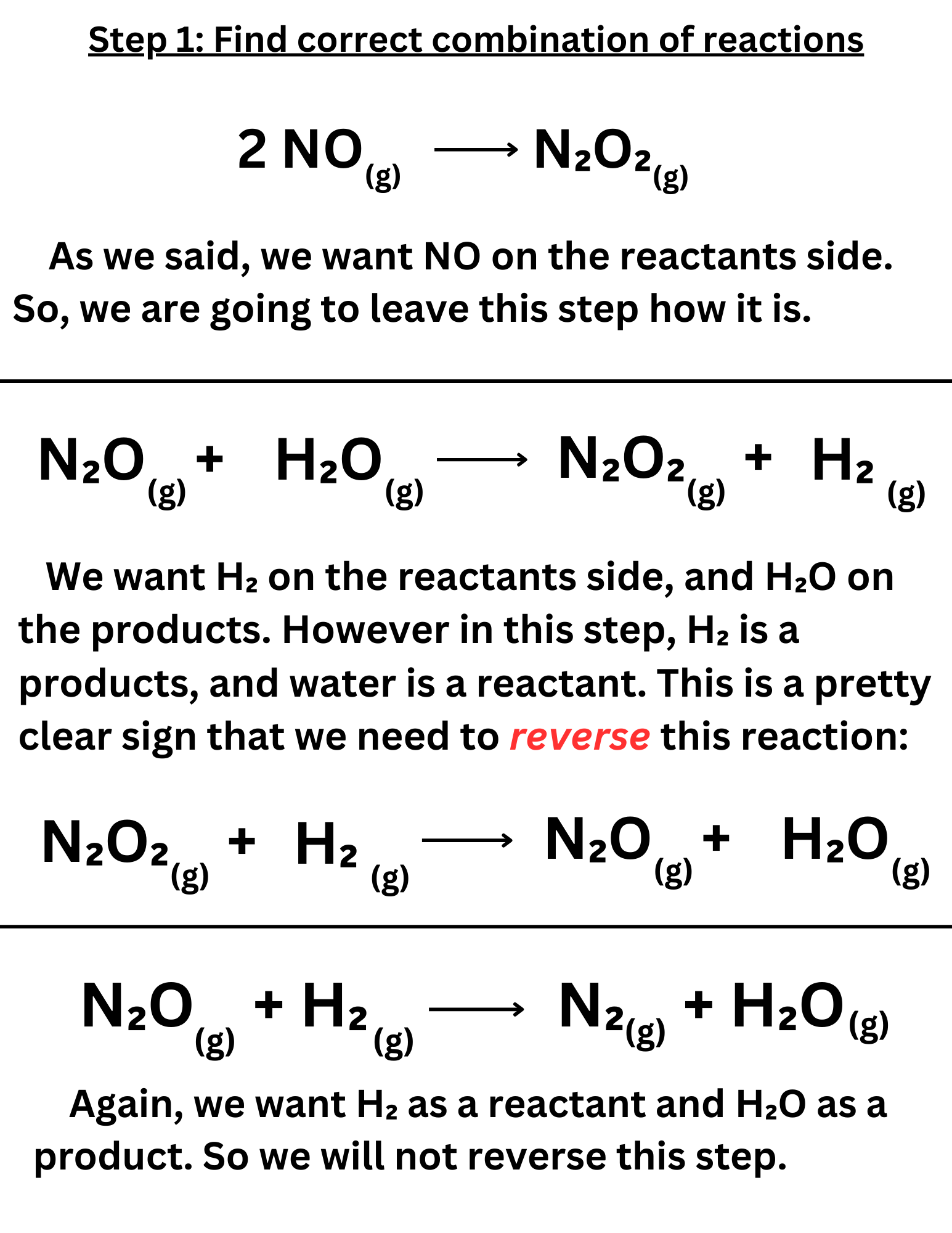
In Hess’ Law problems, you will need to manipulate reactions, flipping reactions and adding steps together. So, it is important to understand how changing a reaction affects the ∆Hᵣₓₙ.
Here’s an example of a Hess’ Law problem ↴

When we combine the individual steps, we want them to add up to the overall reaction. This means we want to set it up in a way where H₂ and NO are on the reactants side, while N₂ and H₂O are on the products side. Make sure that the coefficients are correct.
To achieve this, you may need to reverse some reactions. If you do so, make sure to negate the ∆H.
Let’s quickly verify that our individual steps add up to the overall reaction. Note that when a compound is both a product and reactant, it cancels out.
Then, we’ll add up the ∆H’s of the individual steps to get our final answer.

So -663.4 kJ/mol is our final answer.
Sometimes, even after you get every compound on the correct side, the coefficients will not match the overall reaction. You may need to multiply one of the steps (multiply the coefficients of each compound by a whole number) to get the correct coefficients. As previously explained, if you do this you must also multiply the enthalpy of that step by the same whole number.
