Hybrid Orbitals
When atoms form covalent bonds, their orbitals overlap to form a shared orbital. When an atom bonds with multiple other atoms, it forms hybrid orbitals.
Hybrid orbitals are a combination of orbitals. For example, an sp³ hybrid orbital is a combination of an s-orbital, and three p orbitals. An sp³d² hybrid orbital is a combination of an s-orbital, three p-orbitals, and two d-orbitals.
Here’s what an sp² hybrid orbital looks like ↴

When you are asked to determine the hybridization of an atom in a molecule, you are being asked to determine the hybrid orbitals formed by that atom.
To determine the hybridization of an atom, you need to find its steric number. Remember ↴
Steric Number = (# of atoms directly bonded to the atom) + (# of lone pairs on the atom)
The steric number tells you how many orbitals are involved in the hybrid orbital.
Ex. An sp³ hybrid orbital is a combination of 4 orbitals (1 s-orbital and 3 p-orbitals). So, an atom that is sp³ hybridized has a steric number of 4.
Then, to determine which orbitals are involved, you follow this order ↴

Let’s do an example ↴
Example Problem
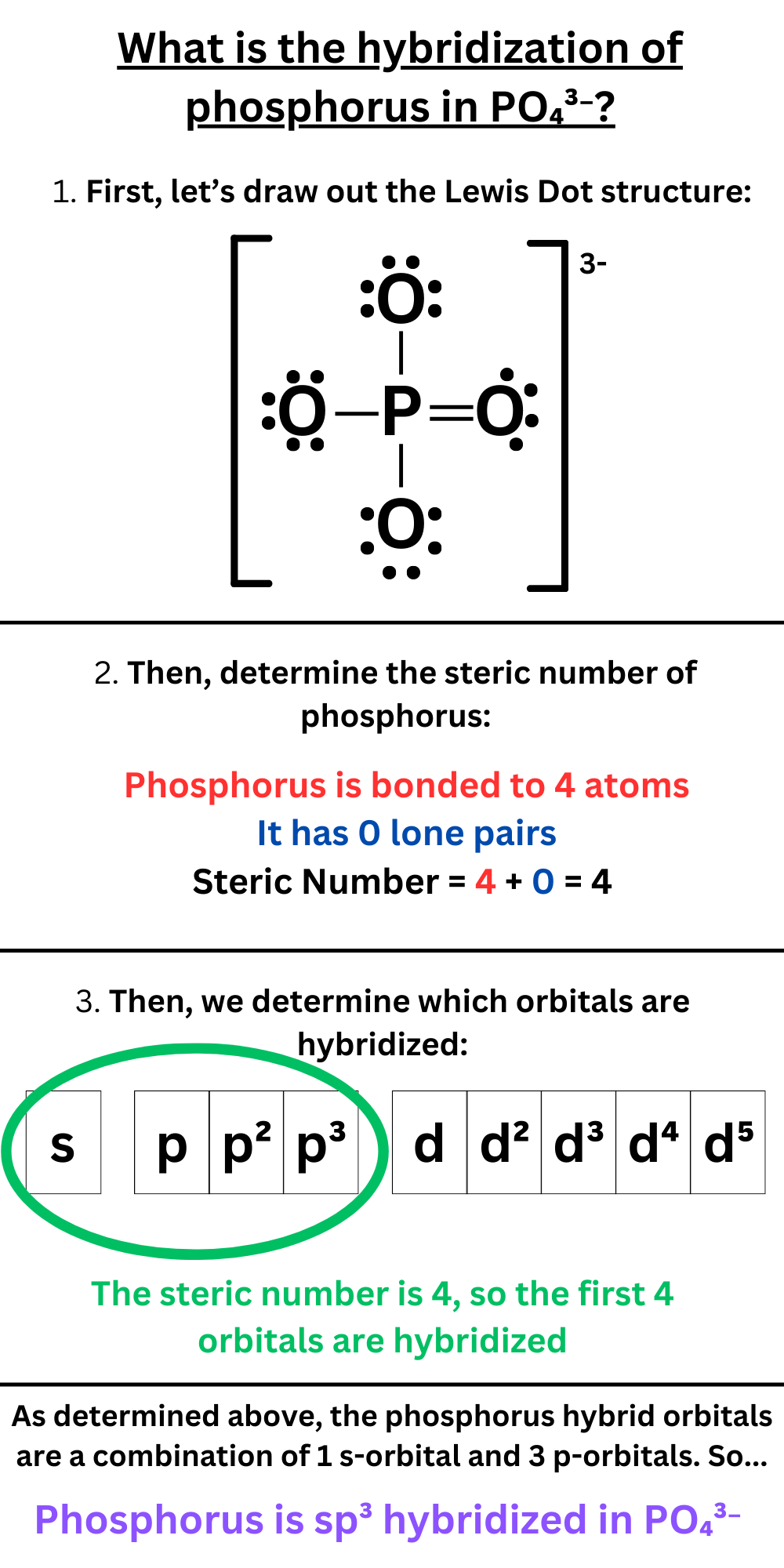
What is the hybridization of phosphorus in PO₄³⁻?
Answer
The more hybrid orbitals involved, the longer the bond. So an sp² orbital is longer than an sp orbital.
The more hybrid orbitals involved, the weaker the bond. So an sp orbital would form a stronger bond with other atoms than an sp² orbital would.
Sigma Bonds
Before, when discussing hybrid orbitals, we talked about how the orbitals of an individual atom combine. Now let’s discuss how the orbitals of two separate atoms overlap when forming a covalent bond.
A sigma bond (σ bond) is formed by orbitals overlapping over the center, known as a “center-center overlap”. Here’s what that looks like ↴

Electrons in a sigma bond are called sigma electrons (σ electrons).
Because of the direct overlapping of orbitals, sigma bonds are the most stable kind of bond.
All single bonds are sigma bonds. In double bonds and triple bonds, only one of the bonds is a sigma bond. The others are pi bonds.
Pi Bonds
Pi bonds (π bonds) are formed by orbitals overlapping side-to-side. This is known as a “side-side overlap”. This is what that looks like ↴

The electrons in pi bonds are called pi electrons (π electrons). When a molecule exhibits resonance, the delocalized electrons will always be pi electrons.
The indirect overlap of orbitals in a π bond causes it to be weaker than a sigma bond.
Every type of bond only contains one sigma bond. In other words, single, double, and triple bonds only contain one sigma bond. All other bonds are pi bonds. Thus, a double bond has one pi bond and one sigma bond. A triple bond has two pi bonds and one sigma bond.”

