The ideal gas law is the heart of the gas laws unit. It is an equation that will need to be used a lot on the AP Exam, so understand it well.
As mentioned in the “Minor Gas Laws” section, it combines Boyle’s Law, Charles’ Law, Avogadro’s Law, and Gay-Lussac’s Law. The ideal gas law states ↴
PV = nRT
– P = pressure in atm
– V = volume in liters
– n = moles of gas
– R = 0.0821 (L • atm)/(mol • K). This is known as the ideal gas constant.
– T = temperature in Kelvin
For this equation, you really have to pay attention to units. If you are given pressure in torr (mmHG), then you must convert to atm first. If you are given the temperature in celsius, then you must convert to Kelvin.
You will see this ideal gas constant (R) in later units. Sometimes it will be 8.314 J/(mol • K), but for gasses it is most helpful to use R = 0.0821 (L • atm)/(mol • K).
Here is how the ideal gas law is a combination of some of the other gas laws ↴

Thus, if we rearrange the ideal gas law so that there is an initial and final state, we can derive the other gas laws from it.

Let’s do some example problems using the ideal gas law.
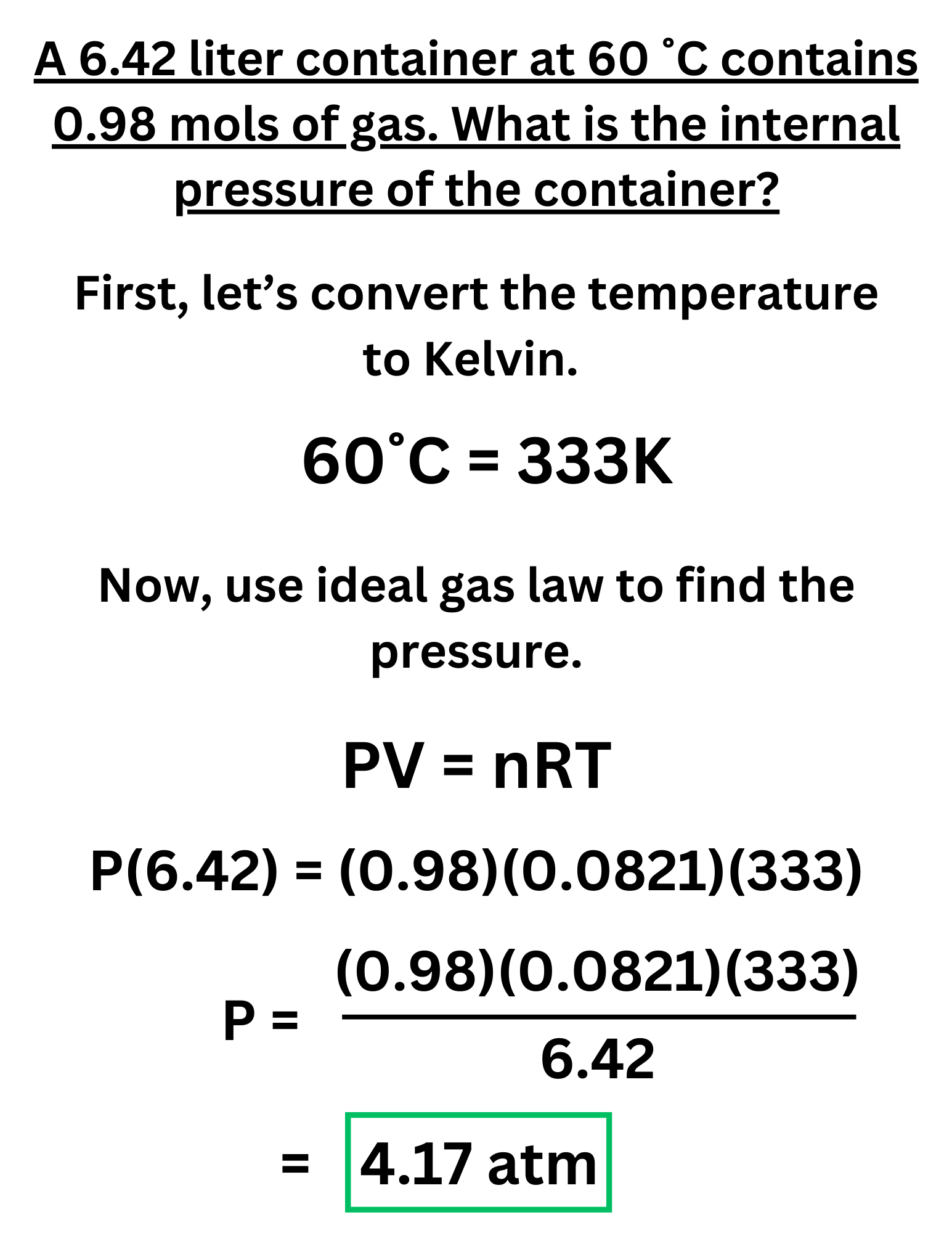
Example Problem #1
A 6.42 liter container at 60 ˚C contains 0.98 mols of gas. What is the internal pressure of the container?
Answer
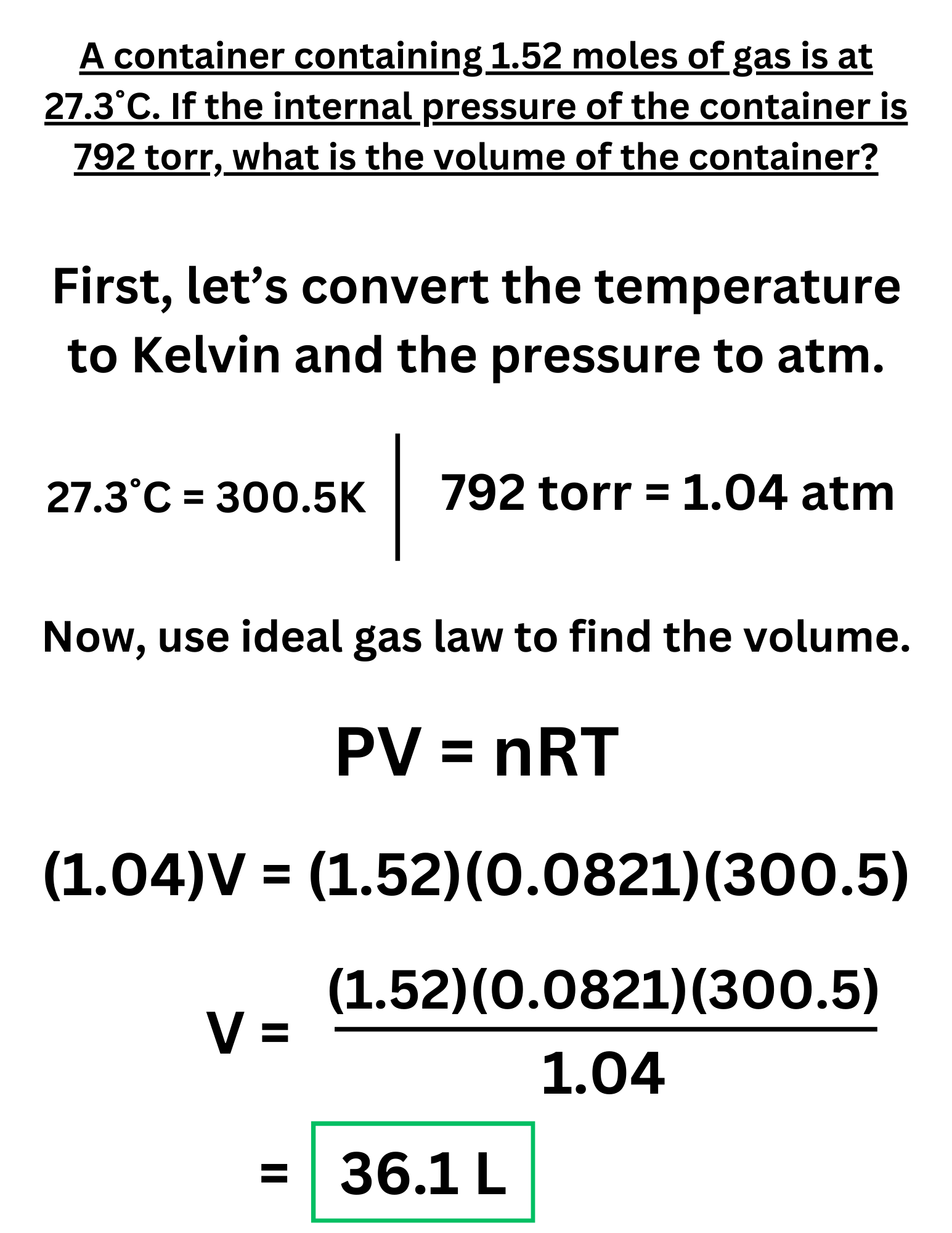
Example Problem #2
A container containing 1.52 moles of gas is at 27.3˚C. If the internal pressure of the container is 792 torr, what is the volume of the container?
Answer
Dirty Pee
We can rearrange the ideal gas law so that it includes molar mass (M), and density (D). This version of the equation may be helpful in some scenarios, but in most cases you will use PV = nRT.
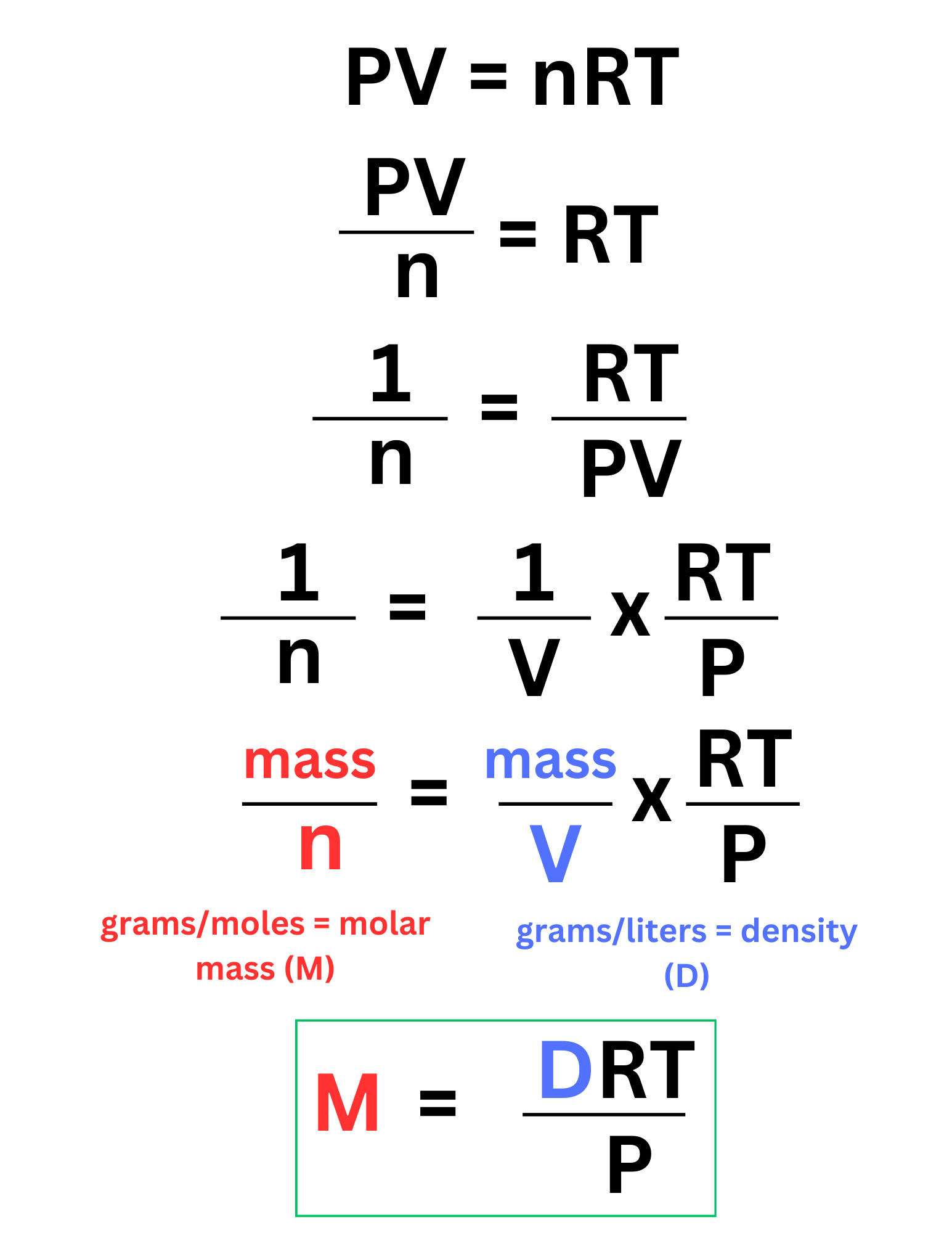
To remember this equation, you can say “Dirty Pee”, which corresponds to DRT/P.
