Ionic Compounds
An ionic compound is a compound formed between a metal and non-metal. Two charged particles (anion and cation), combine to form a neutral molecule.
Essentially, the non-metal completely takes electrons from the metal to become stable. This then forms a non-metal anion and a metal cation. Because opposites attract, the negative non-metal and positive metal come together with. Their charges cancel out to form a neutral compound.
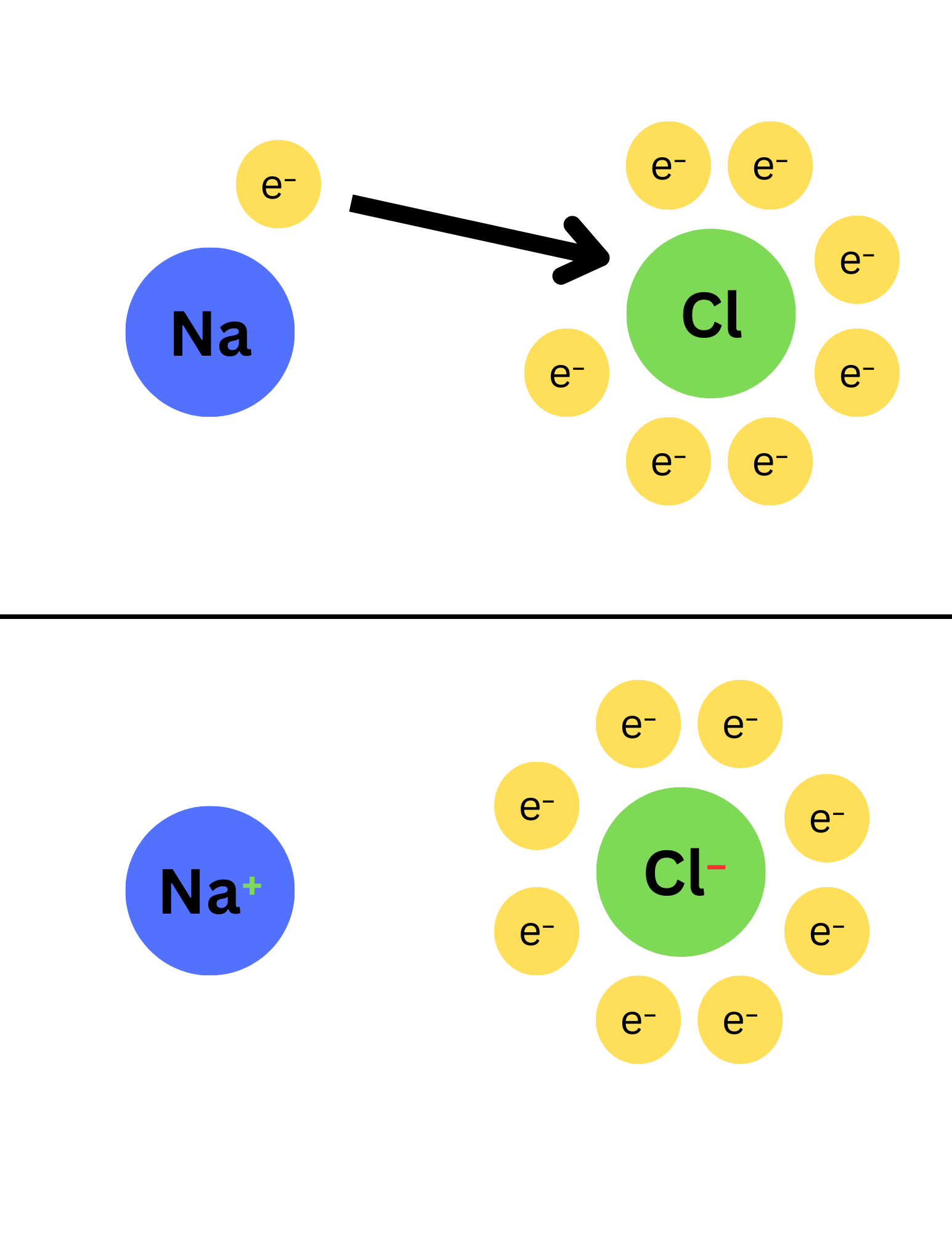
As you can see in the image above, chlorine completely takes the electron from sodium.
The image below shows the ordered lattice structure of ionic compounds. Note how there are not distinct molecules in ionic compounds, rather a large, ordered chain ↴

Molecular/Covalent Compounds
Molecular compounds are made of two or more nonmetals. They have covalent bonds, where electrons are shared. Atoms in a molecular compound are neutral (not charged).
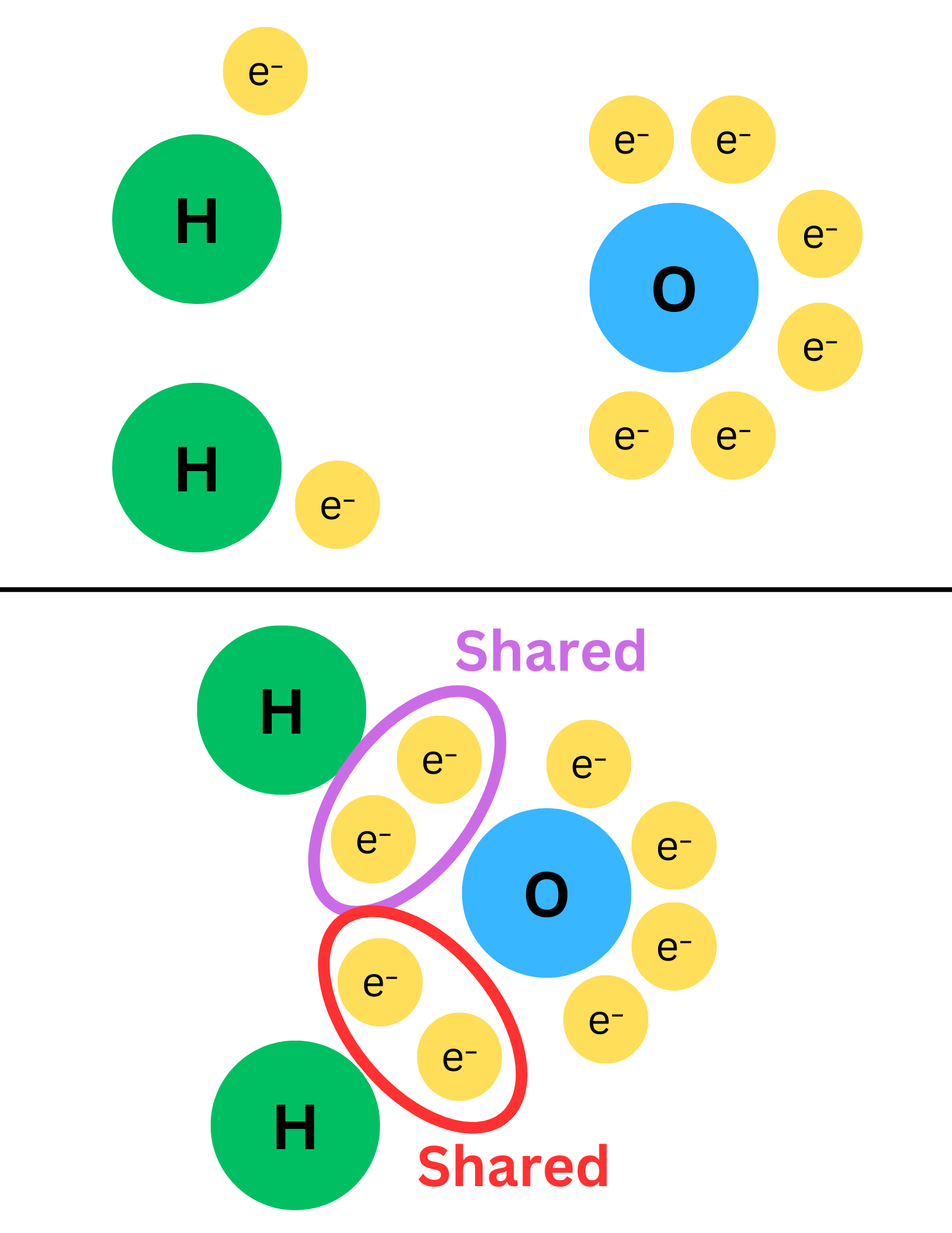
The image above shows that neither oxygen nor hydrogen completely steals each other’s electrons (like ionic compounds do), instead sharing them.
While ionic compounds have a lattice structure, molecular compounds form distinct molecules as shown below.

Polyatomic Ions
Typically, ions are single atoms, like Na⁺, Cl⁻, O²⁻, but there are polyatomic ions, which consist of multiple atoms.
These polyatomic ions can combine with other ions to form ionic substances, so be on the lookout for them.
Memorize this list of common polyatomic ions
– Acetate: C₂H₃O₂⁻
– Ammonium: NH₄⁺
– Carbonate: CO₃²⁻
– Hypochlorite: ClO⁻
– Chlorite: ClO₂⁻
– Chlorate: ClO₃⁻
– Perchlorate: ClO₄⁻
– Cyanide: CN⁻
– Dichromate: Cr₂O₇²⁻
– Bicarbonate: HCO₃⁻
– Bisulfate: HSO₄⁻
– Hydroxide: OH⁻
– Nitrite: NO₂⁻
– Nitrate: NO₃⁻
– Phosphite: PO₃³⁻
– Phosphate: PO₄³⁻
– Sulfite: SO₃²⁻
– Sulfate: SO₄²⁻
