These ideas are used to explain all of the periodic trends, so understand them well.
The Periodic Table
You should already know this, but here’s a reminder ↴
Group: A column in the periodic table.
Period: A row in the periodic table.
We will discuss how certain properties change across periods and up or down groups.
Attraction and Repulsion
Opposite charges attract, and like charges repel. Protons attract electrons, and electrons repel other electrons.
The force of attraction between the positive nucleus and negative electrons is the basis for periodic trends. However, interactions between electrons are also important.
Electrons

Valence Electrons: The outermost shell of electrons in an atom.
Core/Inner Electrons: All non-valence electrons in an atom.
Shielding Effect
The shielding effect is where inner electrons partially block outer electrons from the attraction of the nucleus.
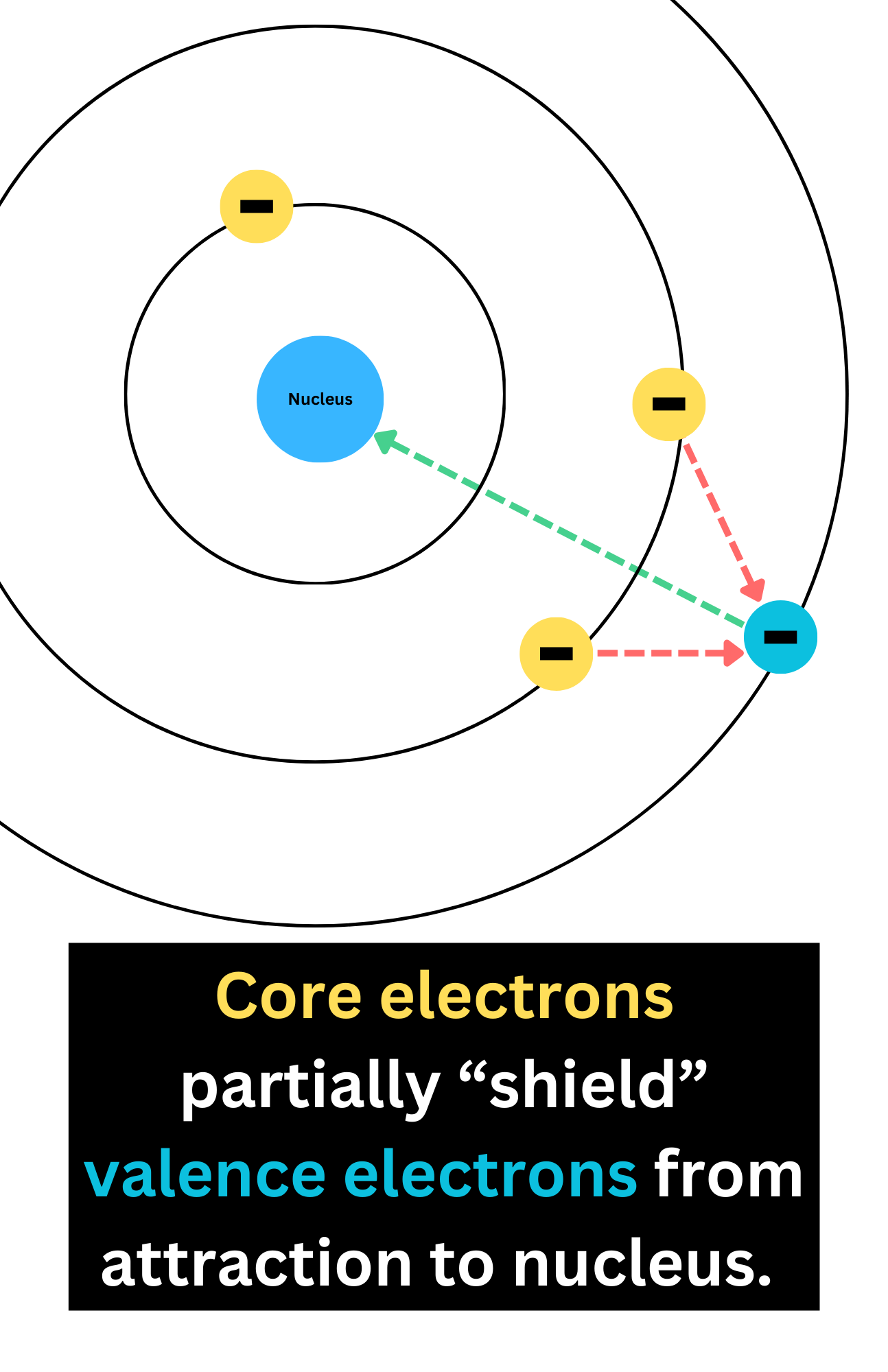
Therefore, electrons farthest from the nucleus experience the least attraction to it.
The more core/inner electrons, the stronger the shielding effect, resulting in a weaker force of attraction between the nucleus and valence electrons.
Note that electrons on the same principal energy level do not shield each other. For example, 4s electrons do not shield 4p electrons, but 3s electrons shield 4s and 4p electrons.
This is also called the screening effect because inner electrons “screen” the outer electrons from the “pull” of the nucleus.
Electron-Electron Repulsion
As aforementioned, electrons repel each other because they have the same charge. This happens between all electrons, even if they are at the same energy level.
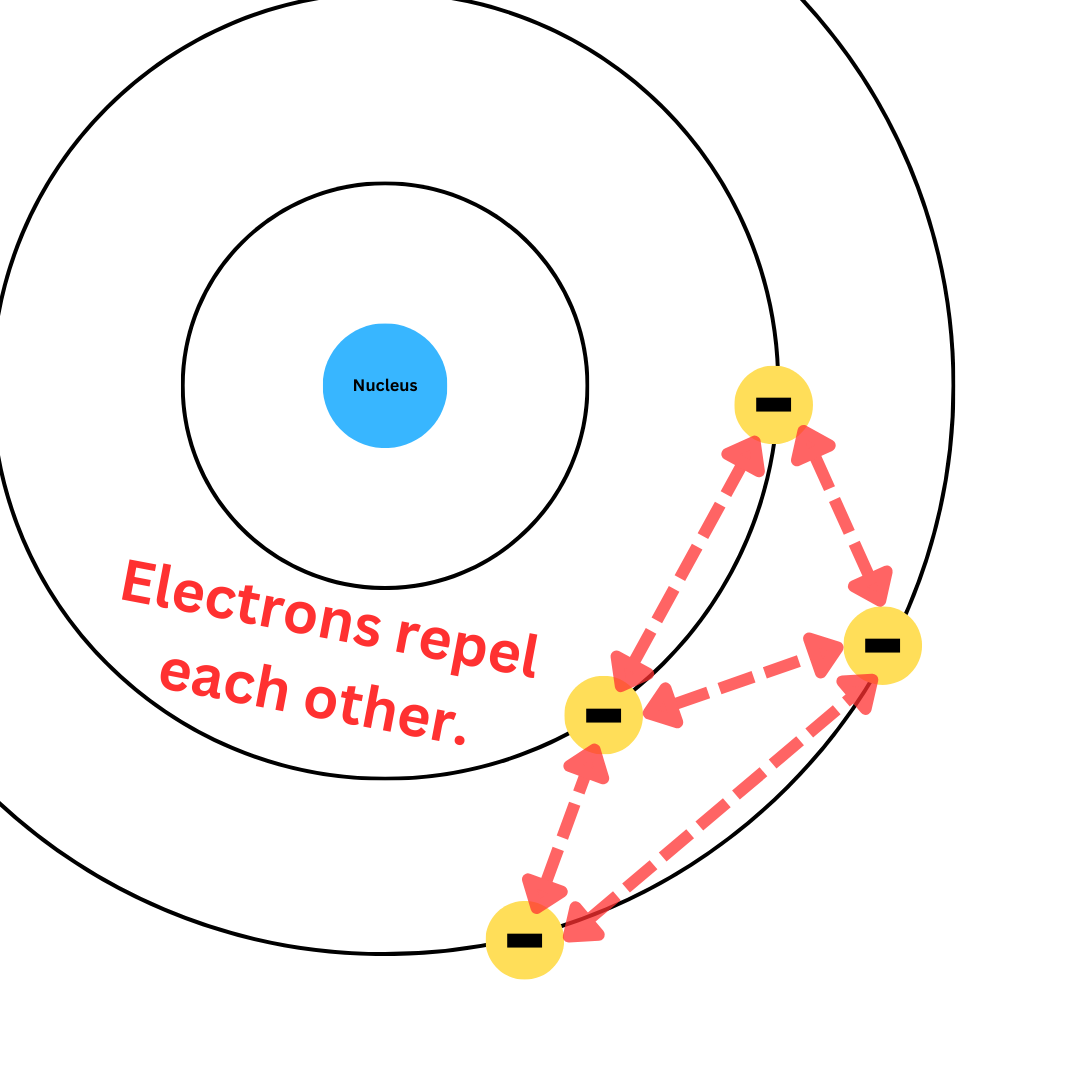
Repulsion can counteract the attraction to the nucleus as valence electrons are pushed away by other electrons. However, electron-electron repulsion is much weaker than the shielding effect.
Effective Nuclear Charge
Effective nuclear charge measures the force of attraction between valence electrons and the nucleus.
Zₑᶠᶠ = Z – S
– Zₑᶠᶠ is the effective nuclear charge
– Z is the number of protons in the atom
– S is the screening/inner electrons
As you can see, this equation takes into account Protons are what attract electrons to the nucleus, so increasing the number of protons increases attraction to the nucleus.
Let’s calculate the Zₑᶠᶠ of bromine. Bromine’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. The 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ electrons are all inner electrons (even though 3d is higher energy than 4s, it is not valence because it has a lower principal energy level). That’s 28 core electrons. Bromine has 35 protons. 35 protons – 28 screening electrons = 7. So Zₑᶠᶠ = 7 for bromine.
It will always work out that the Zₑᶠᶠ = # valence electrons. Our effective nuclear charge for bromine is 7, and bromine has 7 valence electrons.
As you move from left to right across a period, effective nuclear charge increases. The number of protons increases, but the number of screening electrons remains the same, so the valence electrons are more attracted to the nucleus.
