What is a Mixture
A substance made of two or more different pure substances that are not chemically bonded. Essentially, a mixture has different types of molecules in it. The key is that these pure substances are not chemically bonded.
Homogeneous
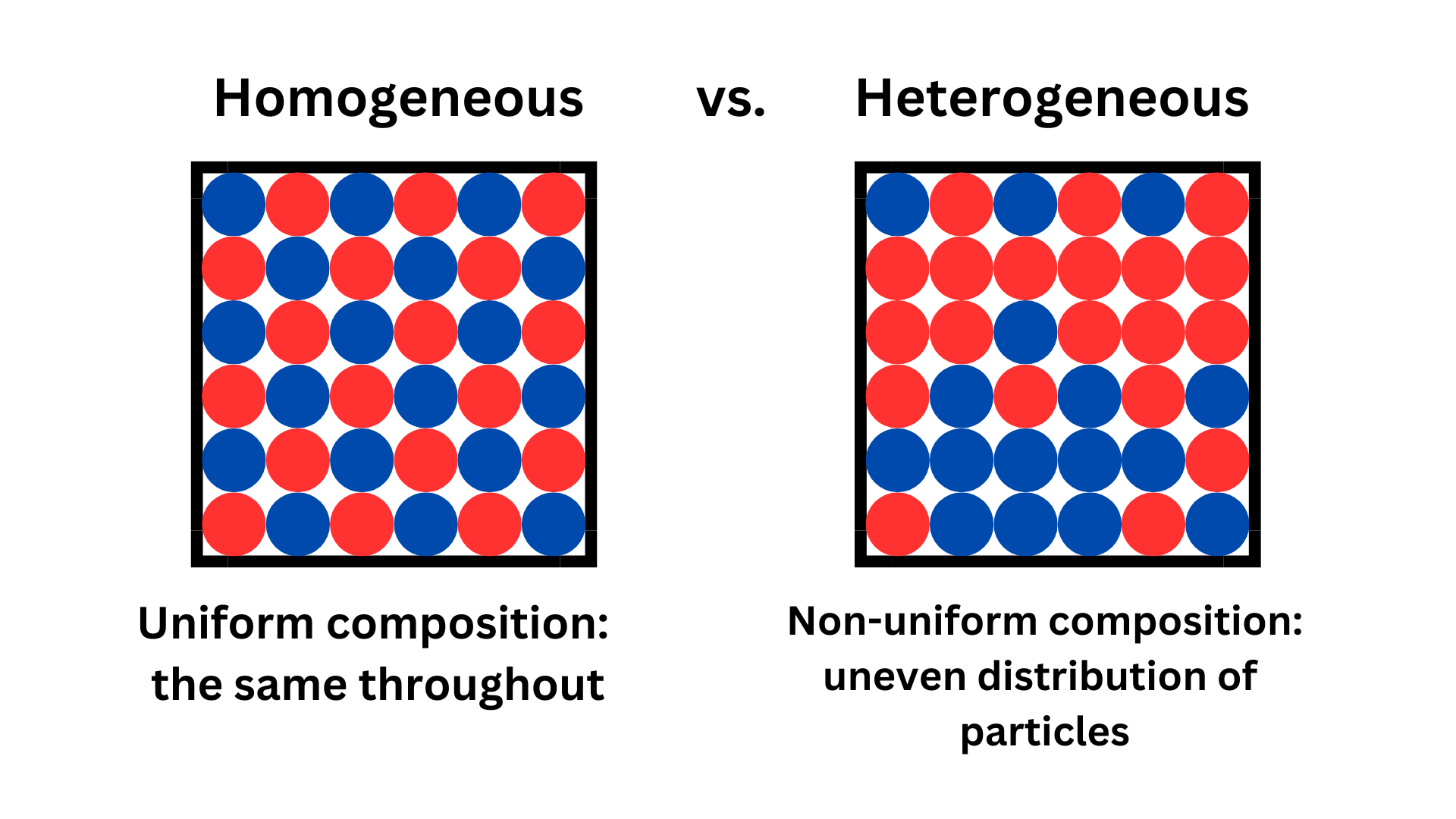
A homogeneous mixture is a mixture that has uniform composition (even distribution). At a first glance, you would not be able to distinguish between components of the mixture. For example, in salt water, you can’t identify the salt in the water because the salt has been distributed evenly amongst the water.
Other examples include brass, an alloy made up of copper and zinc; steel; coffee; blood; etc.
Heterogeneous
A heterogeneous mixture does not have uniform composition. It is not the same throughout. For example, a salad may have more tomatoes on one side than the other. Or, your cookie may have more chocolate chips in certain areas.
Other examples include sandwiches, a bowl of cereal and milk, a bowl of fruit, etc.
Alloys
Alloys are a way of classifying a homogeneous mixture of two or more metals. They can also include non-metals but must have a metallic base. Atoms in alloys are tightly packed together.
For example, brass is an alloy composed of copper and zinc. Steel is an alloy composed of iron and carbon.
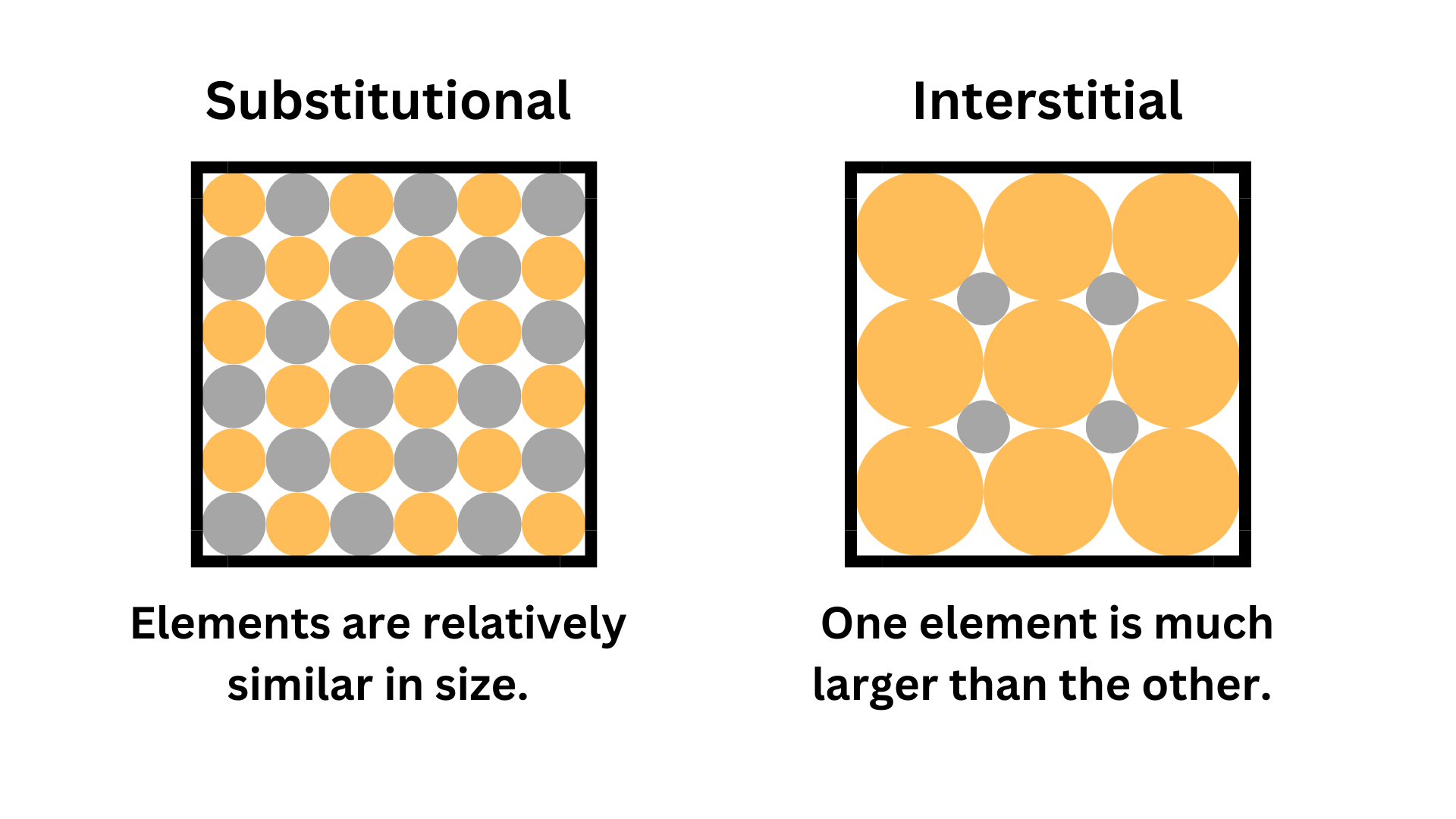
Substitutional: Atoms in the alloy are around the same size.
Interstitial: One component of the alloy is much smaller than the other component. The smaller atoms fill the tiny gaps between larger atoms.