Aqueous Reactions
Aqueous reactions take place in water. Two ionic compounds are placed into water. They ionize, meaning they split up into their cations and anions. The ions of these compounds may react to form a precipitate (defined below).
These compounds cannot react without being split into their ions, which is why these reactions can only take place in water.
Aqueous State: The state of being dissolved in water. Denoted by the subscript “(aq)”.
Spectator Ions: Ions that don’t participate in the reaction, staying the same before and after. They remain in an aqueous state throughout.
Precipitate: Solid product formed via an aqueous reaction.
Net ionic equations describe aqueous reactions, and balancing them is slightly different.
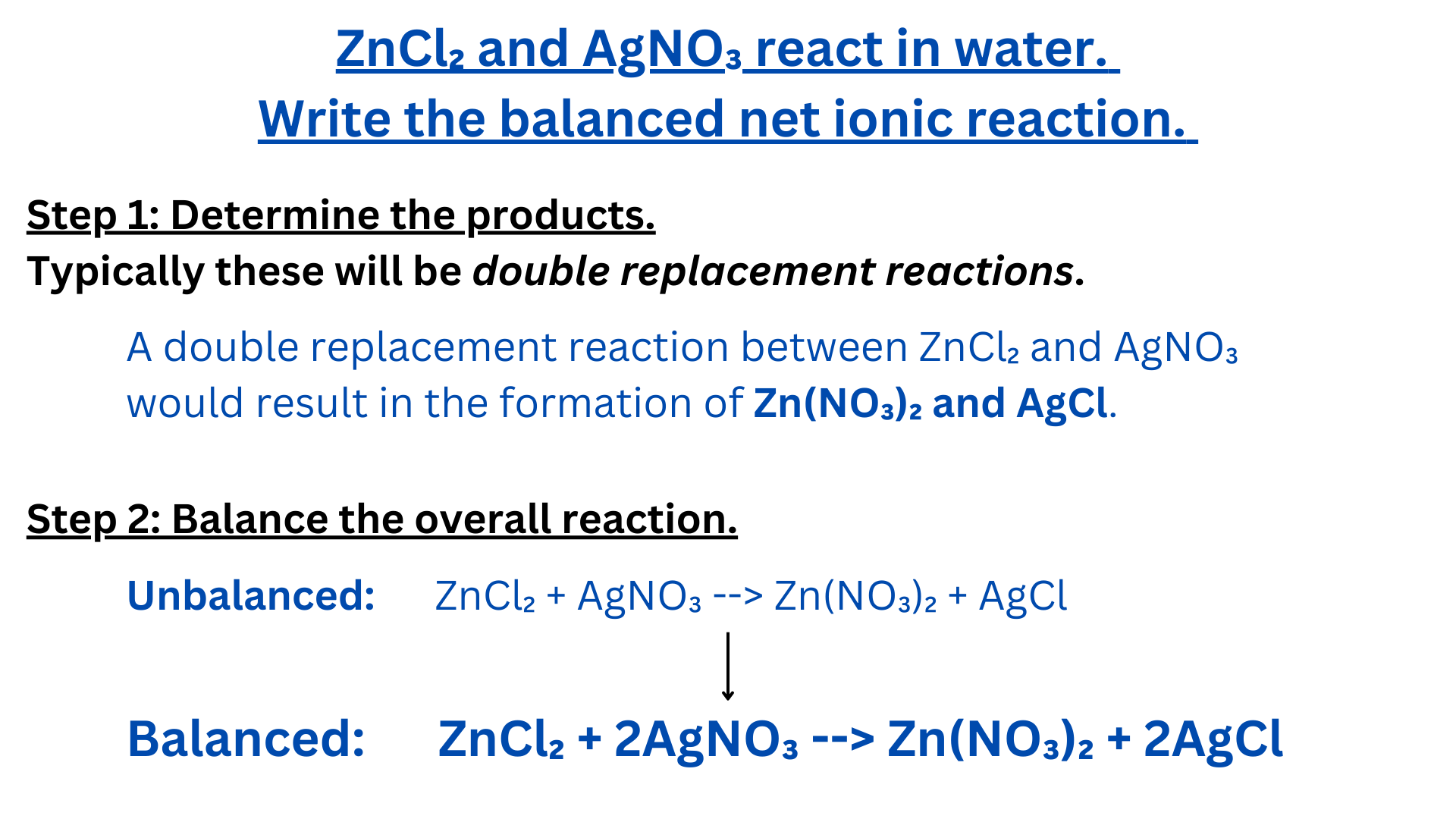
Writing Net Ionic Equations
We are going to explain how to write net ionic equations by walking through an example ↴