Atomic Stability
Noble gasses are the most stable elements because they have a full valence shell. To obtain a full valence shell, most atoms need 8 valence electrons (reminder: valence electrons are the outermost electrons).
Atoms want to have a noble gas configuration (8 valence electrons) to become stable.
Determining Common Ion Charges
The common ion charges can be determined using the columns in the periodic table.
To have 8 valence electrons, atoms either gain or lose electrons. This causes them to either become anions or cations. We can look at how many valence electrons an atom has to determine the charge of its ion.
You have seen groups on the periodic table labeled 1-18, but there is another way of writing them. Sometimes you will see group numbers with an “A”, (ex. Group 1A, Group 2A). This classification goes from 1A – 8A and excludes the transition metals, as shown in the image below ↴
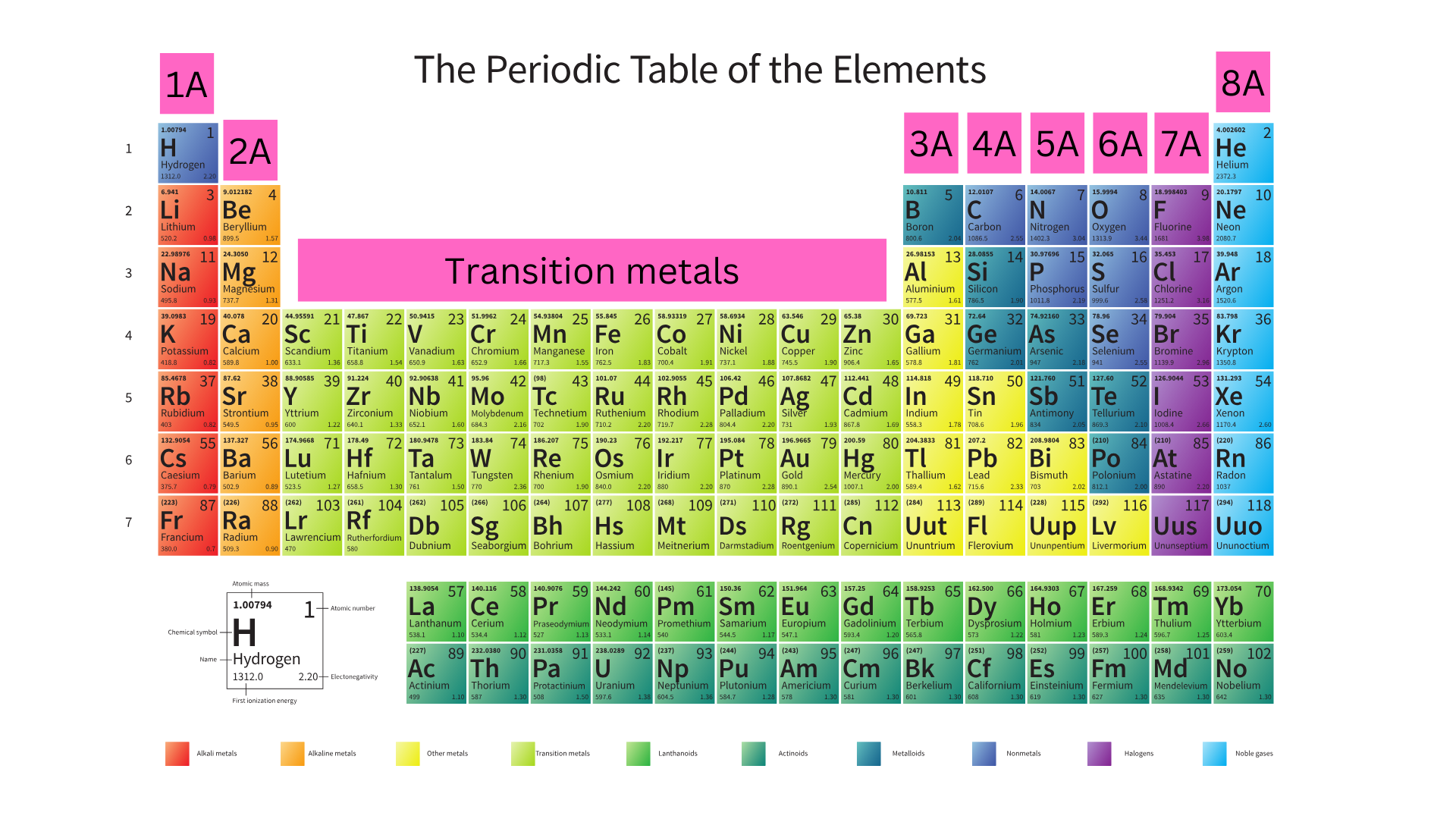
Column 1A has 1 valence electron, column 2A has 2 valence electrons, column 3A has 3 valence electrons, 4A has 4 valence, 5A has 5 valence, 6A has 6 valence, 7A has 7 valence, and 8A has 8 valence.
All atoms will do the least amount of work to reach stability.
For column 1A – 3A, it is easier for them to lose all of their valence electron(s) as opposed to gaining 5, 6, or 7 electrons. This makes their full inner shell become the valence shell, creating a full valence shell. Losing electrons causes these atoms to be cations.
For column 5A – 7A, it is easier for them to gain valence electron(s) (so that they have 8), rather than losing 5, 6, or 7 electrons. Gaining electrons causes them to become anions.
Column 4A could either gain 4 or lose 4 electrons.
Based on how many electrons an atom would gain/lose to become stable, we can determine its charge.
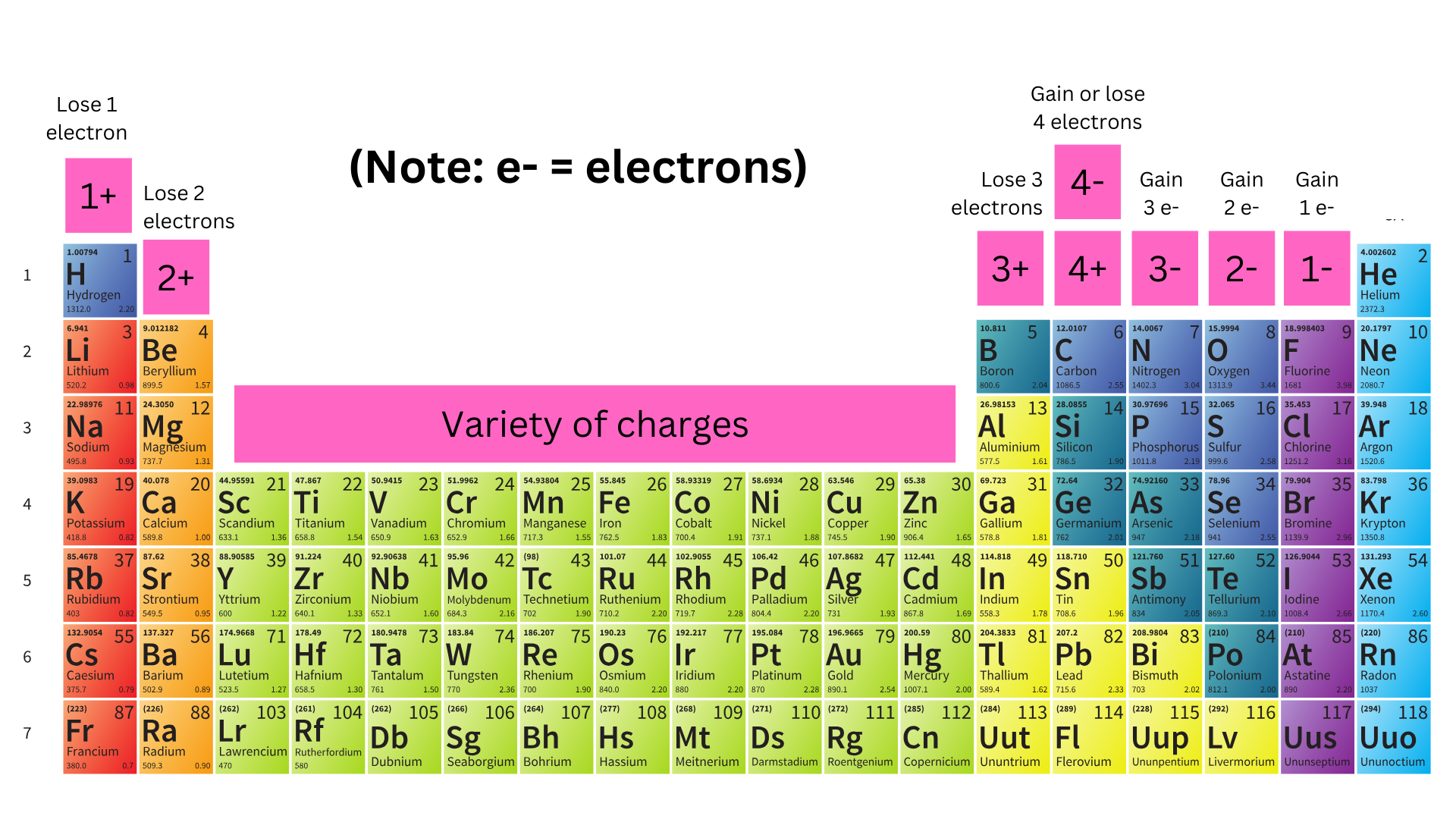
Transition metals are a little weird. They don’t necessarily need 8 valence electrons (you will learn more about this in later units). They have extra spaces for valence electrons, so they can exhibit a range of charges. For example, iron can be Fe²⁺ or Fe³⁺.
Also note that hydrogen can either have 0 valence or 2 valence electrons to be stable. Sometimes you will see H⁺, but when bonded with a metal, hydrogen often becomes H⁻.
