What is an Orbital
Electrons orbit around the nucleus in 3D patterns. People often confuse orbitals with orbits. An orbit is the exact path that the electron takes. Orbitals describe the space that an electron could be in. It is impossible to know exactly where the electron is, but orbitals show where the electrons most likely are.
Each orbital in an atom can hold two electrons. There are different orbital shapes, and most of the time, atoms will have multiple orbitals of the same shape.
Energy levels can sometimes be referred to as “shells,” and orbitals can be referred to as “subshells.”
s orbital

This is the lowest energy orbital. It has a spherical shape. Each energy level has one s-orbital. This means that each energy level can hold a maximum of two electrons with s-orbitals.
p orbital
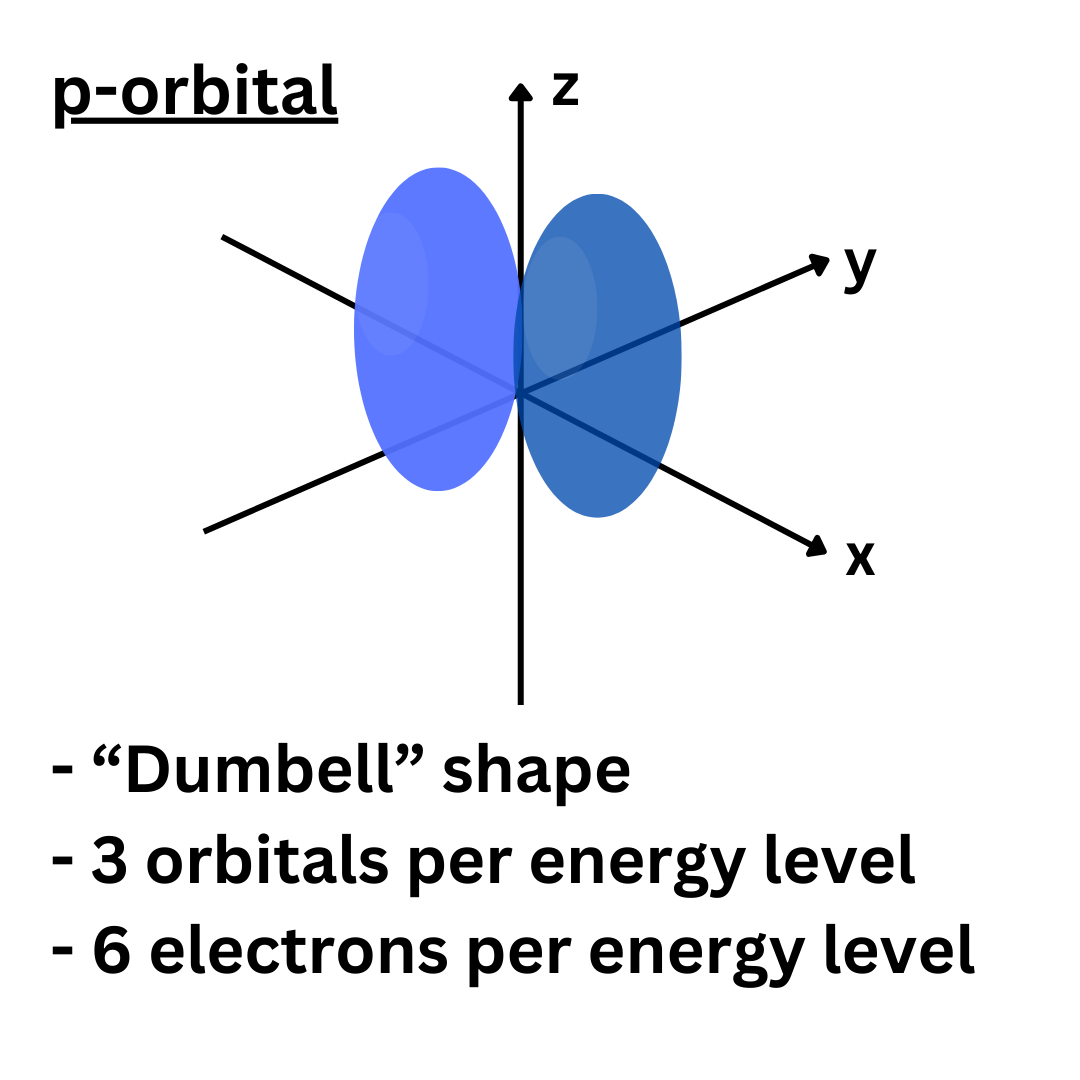
This is the second lowest energy orbital. It is described as having a “dumbbell” shape. The first energy level has no p-orbitals, but the others have three. This means that each energy level can hold a maximum of six electrons with p-orbitals.
d orbital
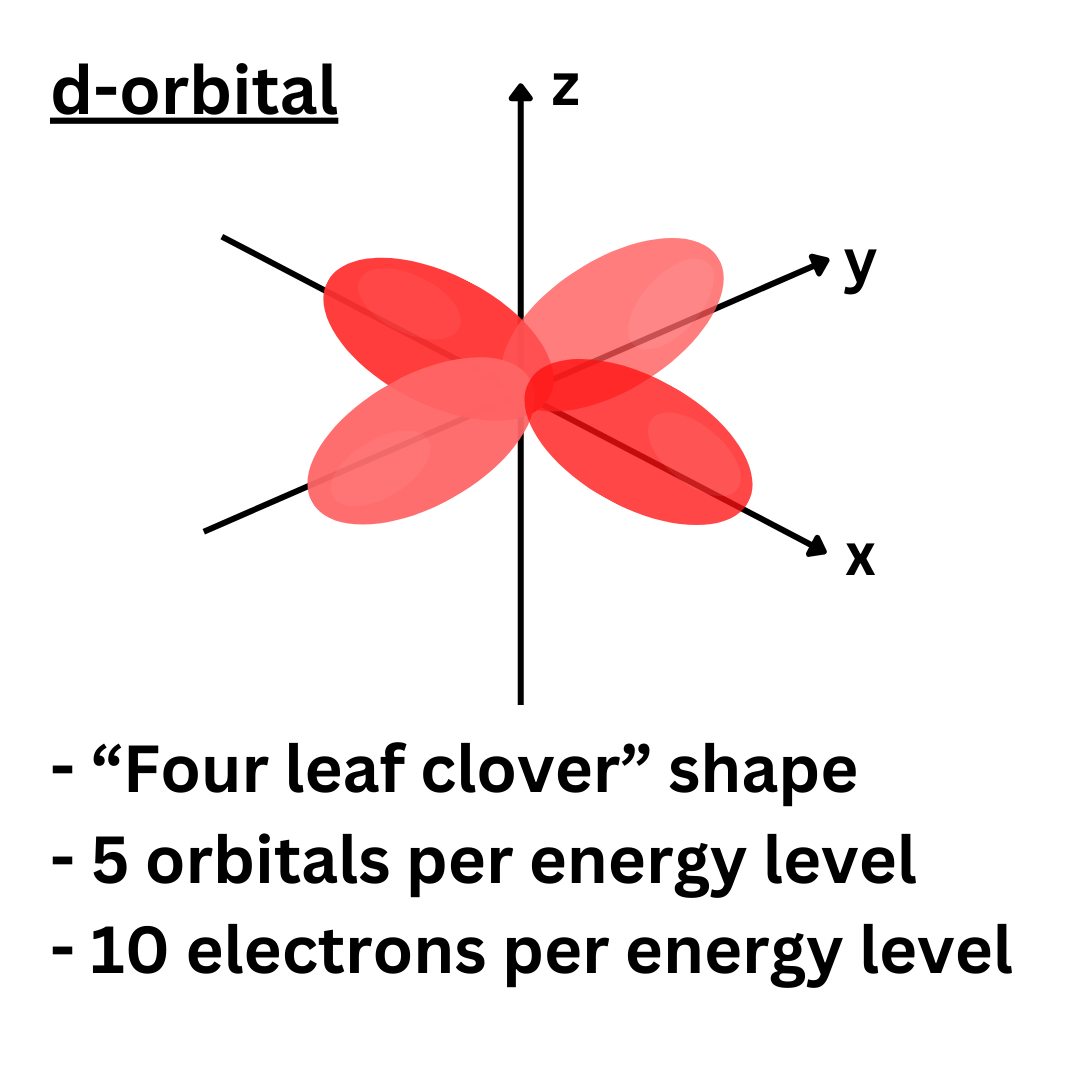
This is the second highest energy orbital. The first and second energy levels have no d-orbitals, but the others have five. This means that each energy level can hold a maximum of ten electrons with d-orbitals.
f orbital

This is the highest energy orbital shape. The first three energy levels do not have an f-orbital, but the others have seven. This means that each energy level can hold a maximum of fourteen electrons with f-orbitals.
Energy
As mentioned, in terms of energy, s < p < d < f. This always holds true within an energy level, but is often not true across different energy levels. For example, an s-orbital in the second energy level (n = 2) has more energy than a p-orbital in the first energy level (n = 1), even though p > s.
