You can draw the Lewis Dot of some molecules in multiple different ways. These rules will help you choose the way that is more correct.
Not all structures are equally stable. The preferred Lewis Dot structure is the one that is most stable.
Rule 1: The sum of the formal charges of the individual atoms must equal the overall charge of the molecule. We’ve discussed this rule many times before, and it is the most important one.

Rule 2: If needed, the more electronegative atom should carry the negative formal charge. The key words are “if needed.” If you have to give an atom a negative formal charge, it should be the more electronegative atom.

Rule 3: For a neutral molecule, it is preferred that the formal charges of all atoms are 0 (rather than one atom with +1 and one atom with -1).
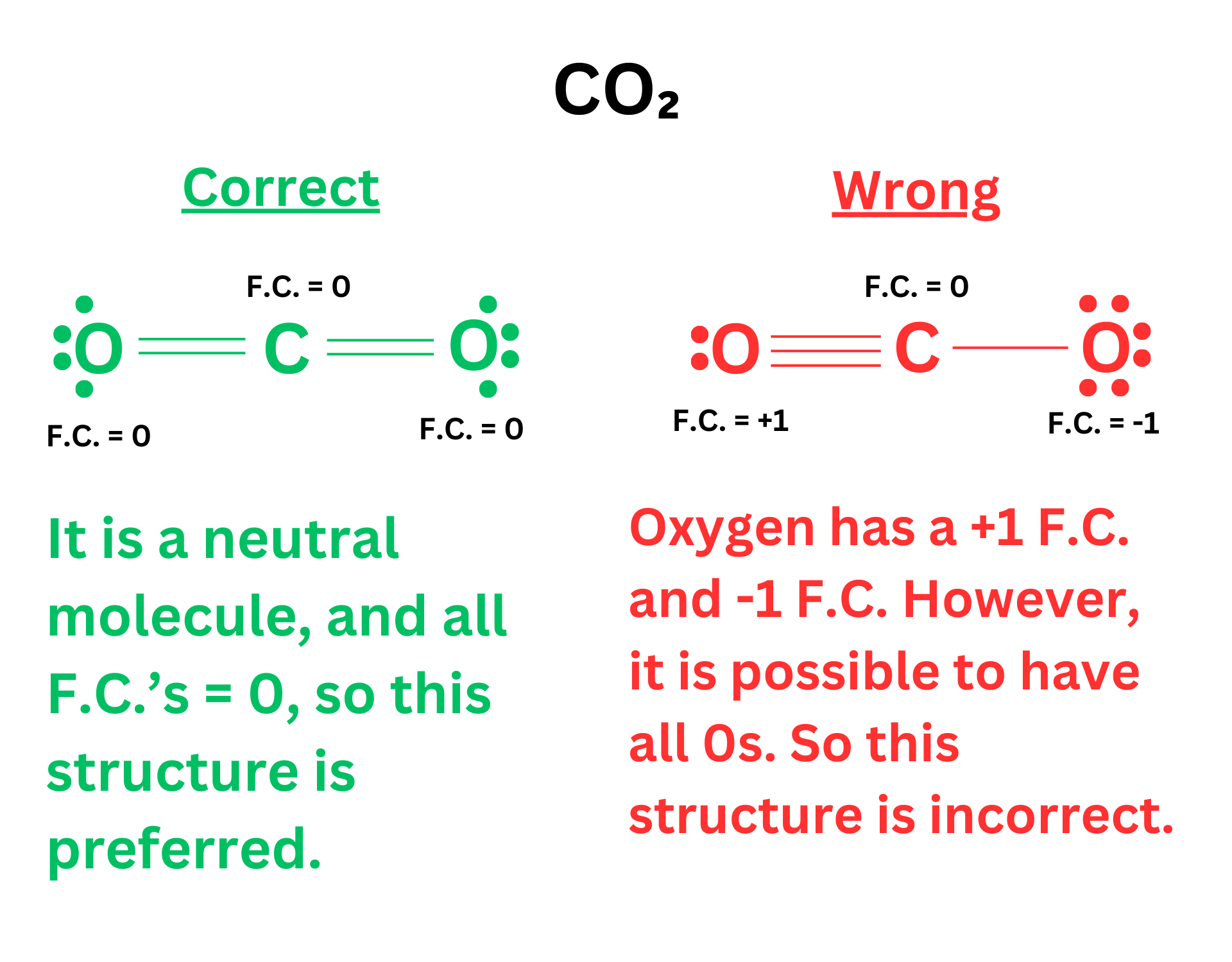
However, sometimes it is impossible to follow this rule. CO is a good example ↴
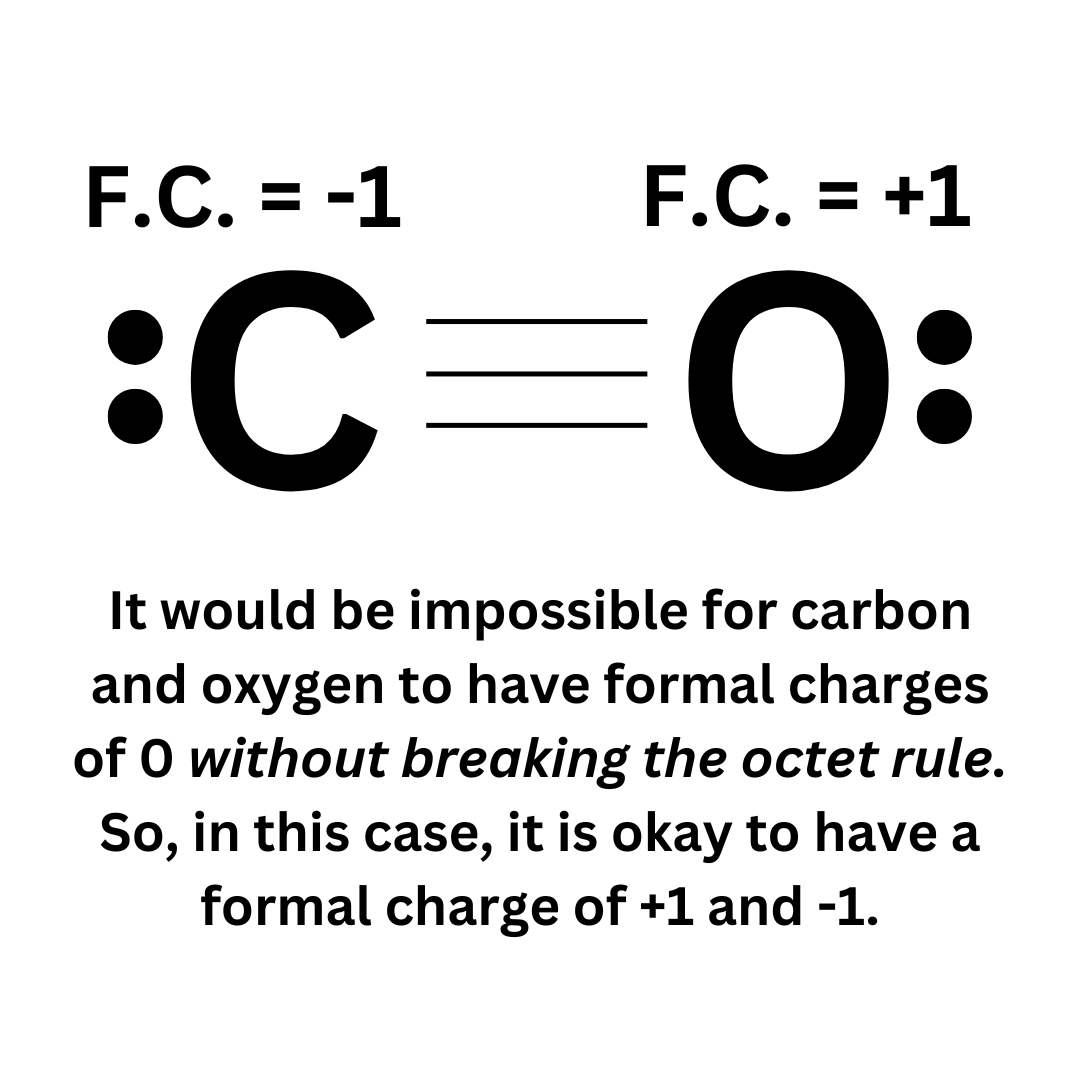
It is impossible to draw this neutral molecule so that all atoms have a formal charge of 0. In that case, it is okay to break this rule. Though, if possible, you must follow this rule.
