What is Redox
Redox reactions involve a transfer of electrons. The word “redox” is a combination of “reduction” and “oxidation”. Those two processes are a package: one cannot occur without the other.
Reduction: The process in which an element gains electrons.
Oxidation: The process in which an element loses electrons.
The reason both processes need to occur together is because when an atom is oxidized, the lost electrons have to go somewhere. Another atom has to gain those electrons and get reduced. So a redox reaction involves one atom being oxidized, and another being reduced.
Reducing Agent: The atom that reduces another atom. The reducing agent is oxidized, meaning that it reduces something else.
Oxidizing Agent: The atom that oxidizes another atom. The oxidizing agent is reduced, meaning that it oxidizes something else.
For oxidizing and reducing agents, it may seem sort of backwards. The oxidizing agent does the oxidizing of another atom, so it gets reduced. The reducing agent does the reducing of another atom, so it gets oxidized.
Oxidation Numbers
Oxidation numbers are used to track the electrons gained or lost by an atom throughout redox reactions. Keep in mind that an atom’s oxidation number does not represent its actual charge. It often will be the same as the charge of the ion/atom, but this is not always the case.
For example in H₂O, hydrogen has a +1 oxidation number, but because water is a molecular compound, the hydrogen molecules are neutral (no actual charge).
In reduction, an atom’s oxidation number decreases (becomes more negative), indicating that it has gained electrons.
In oxidation, an atom’s oxidation number increases (becomes more positive), indicating that it has lost electrons.
A tip to remember this is that in reduction, the oxidation number is being “reduced”, becoming more negative.
Here are rules for calculating oxidation numbers (if they are confusing, look at the example at the end) ↴
Note that O.N. means oxidation number
1. In a neutral molecule, the O.N.’s of its atoms must add up to 0
2. In a polyatomic ion, the O.N.’s of its atoms must add up to the charge of the ion
3. A neutral, free, element has an O.N. of 0 (this also goes for diatomic elements like oxygen, where in O₂ it’s O.N. is 0)
4. A monatomic ion has an O.N. equal to its charge
5. Oxygen almost always has an oxidation number equal to -2 (except in O₂ and peroxides)
6. Fluorine always has an oxidation number equal to -1
7. Hydrogen has an O.N. equal to +1, unless it is with a metal, where it is -1.
There are a lot more rules that cover very specific situations, but by learning these rules, you should be fine for most problems.
It is important to understand how to calculate oxidation numbers, because you can look at how they change throughout a redox reaction. As mentioned before, if the O.N. increases, the element has been oxidized. If it decreases, it has been reduced.
Oxidation Number Examples
Example Problem #1
What is the oxidation number of S in SF₄?
Answer

Example Problem #2
What is the oxidation number of Mn in MnO₄⁻?
Answer

Example Problem #3
What is the oxidation number of K in KClO₄?
Answer

Example Problem #4
What is the oxidation number of F in F₂?
Answer

Example Problem #5
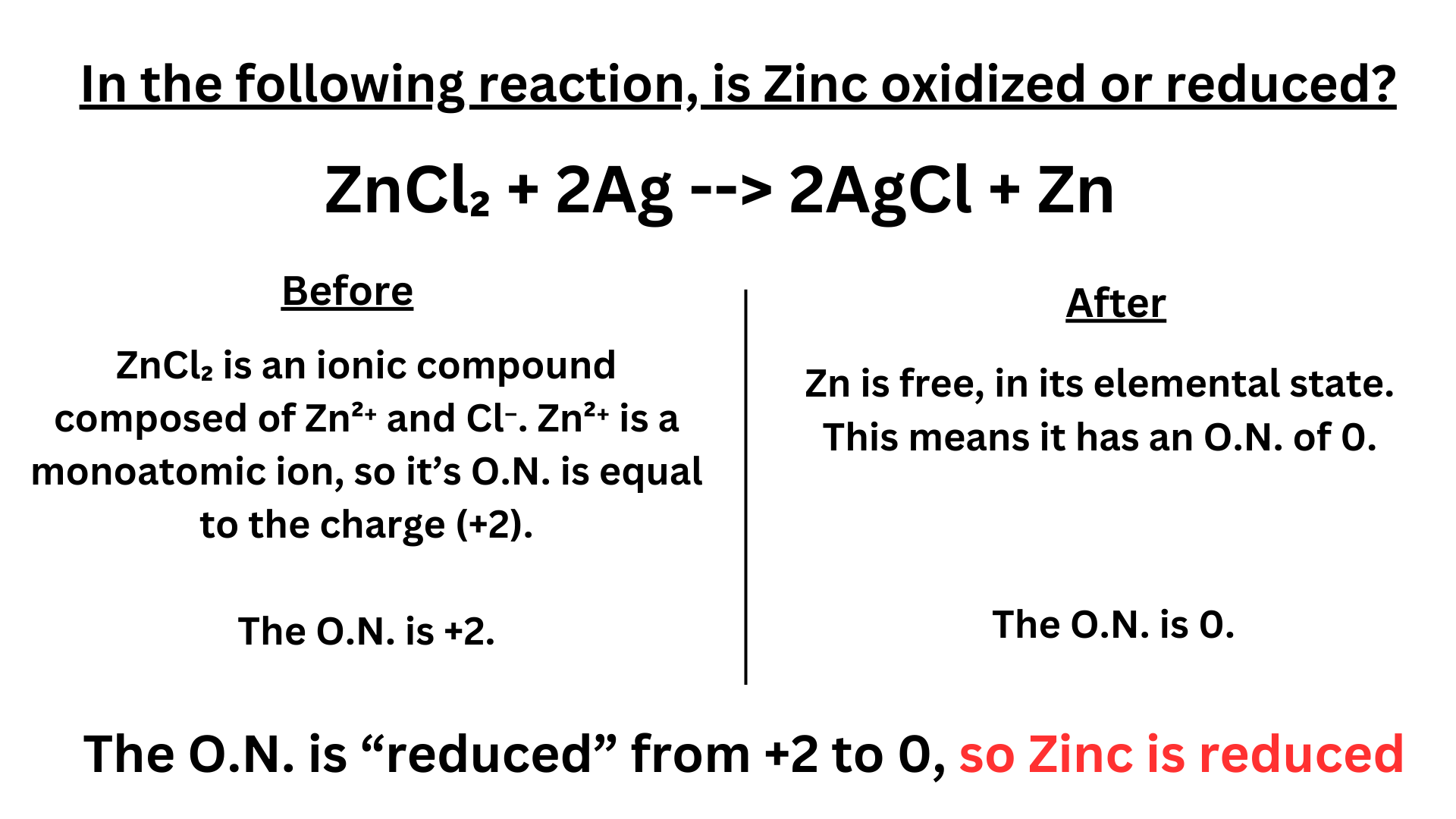
In the following reaction, is Zinc oxidized or reduced? ↴
ZnCl₂ + 2Ag –> 2AgCl + Zn
Answer