Importance
These methods are used to separate components of a mixture. Scientists use them to extract one substance from a larger mixture of unwanted substances. It is important to understand when you can/should use each type of method.
Filtration
Filtration is used to separate substances using a filter, which only allows one substance in the mixture to pass through. Often, the filter has small holes, but other types, such as paper filters, can also be used. The key is that one substance can pass through while the other cannot.

For example, when you drain pasta into a colander, the water is allowed through, but the pasta is not. This separates the mixture of water and pasta.
Filtration can be used to separate substances that have a large difference in size. It cannot separate solutions.
Distillation
Distillation is used to separate two or more different, liquid substances in a mixture. Every substance has a different boiling point. Distillation uses this to boil certain parts of the mixture (which are later recondensed into other containers), while other parts of the mixture remain liquid. Heat is continuously added to the mixture until the substance with the lowest boiling point begins to boil. The other substance will not boil, because its boiling point hasn’t been reached. The process then continues on until the substance with the highest boiling point is all that remains in the initial container. The other substance(s) have been collected in other flasks.

Distillation can separate substances with a large difference in boiling points. If the boiling points are too similar, both liquids will boil at the same time. They will not be separated.
Chromatography
Separating substances based on their attraction to other substances. In paper chromatography, there is a stationary phase (typically paper), and a mobile phase (typically water), which travels through the stationary phase. The mobile phase pulls components of the mixture (typically dyes), separating them.
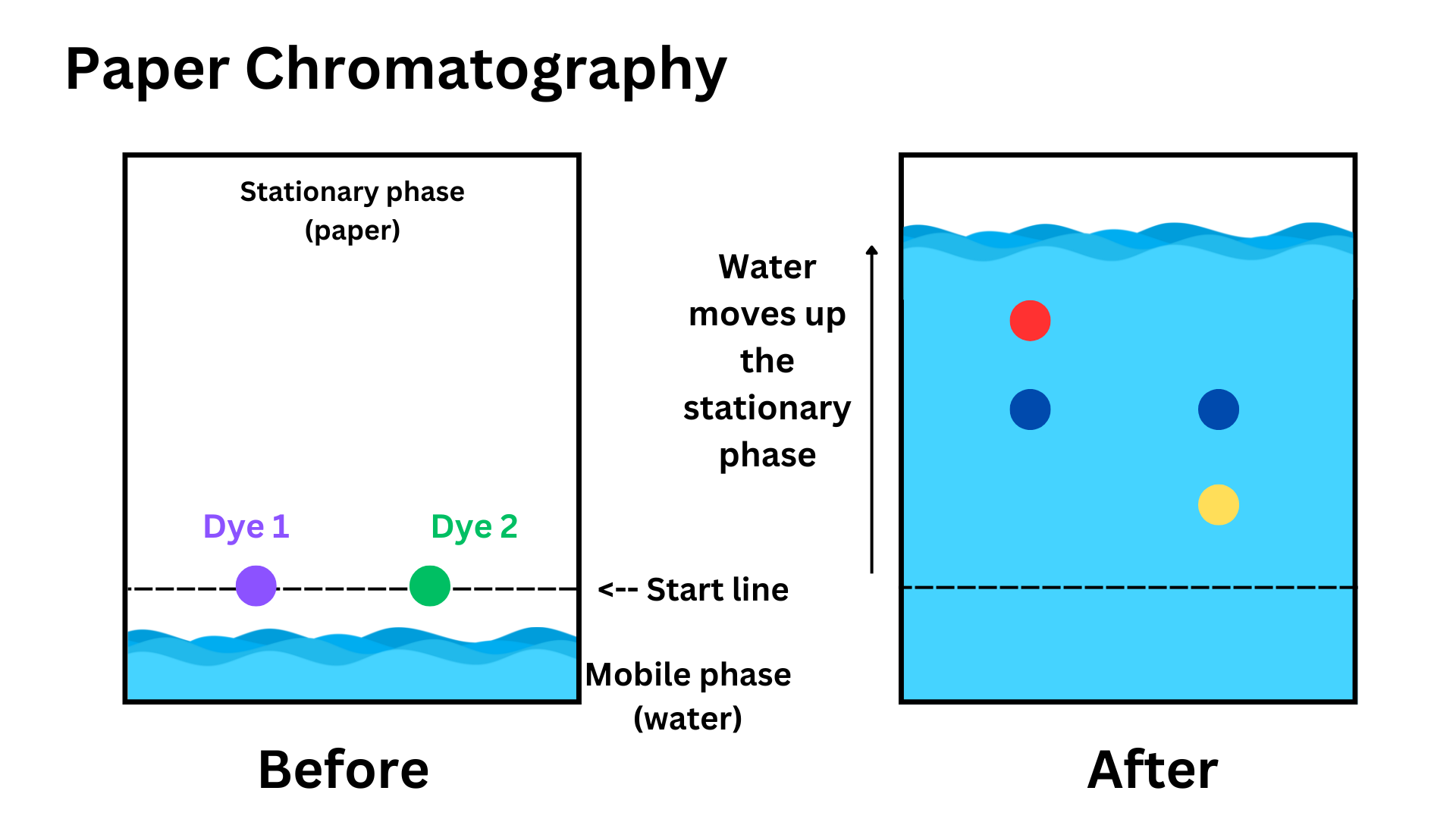
The water (mobile phase) moves up the paper (stationary phase), and certain parts of each dye are more or less attracted to the water. The red component moves up a lot because it is very attracted to the water. The yellow component of dye 2 barely moves because it is not very attracted. This difference in attraction separates the dyes into simpler components (separating the mixture).
