Water is the most important solvent in chemistry. Certain substances can or can’t dissolve in water. These rules, explaining which substances are soluble in water, will help you for the rest of the year, so know them well.
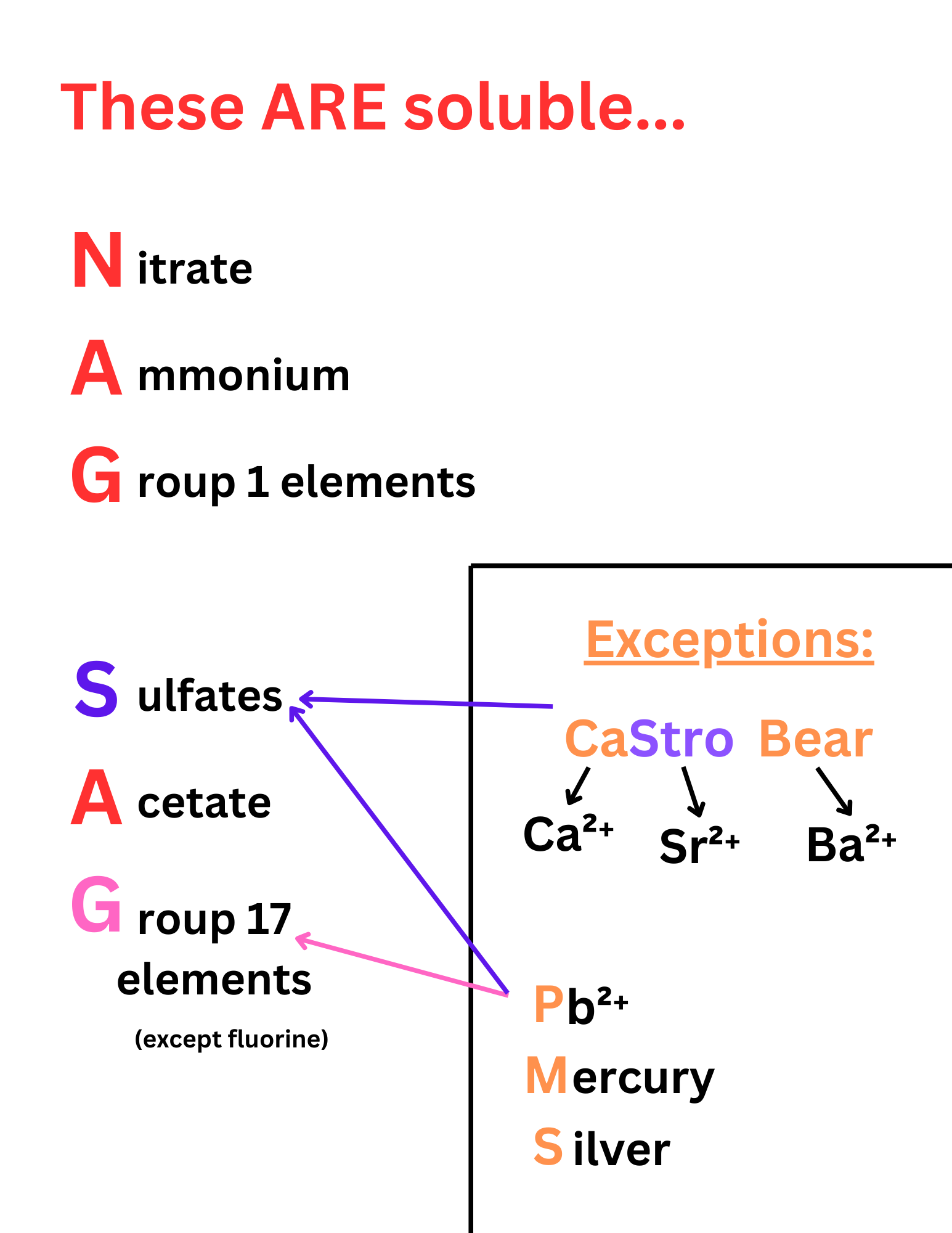
NAG SAG
NAG SAG is an acronym for substances that are extremely soluble in water. If you see these ions in an ionic compound, then that compound is most likely soluble.

PMS & Castro Bear
PMS and Castro Bear stand for exceptions to NAG SAG.
PMS: These ions make sulfates and group 17 elements insoluble ↴
P = Pb²⁺ (lead)
M = Mercury
S = Silver
CaStro Bear: These ions make sulfates insoluble ↴
Ca = Calcium
Stro = Strontium
Bear = Barium
CroSH CarSt FluPh
This mnemonic device helps to remember substances that are very insoluble in water. However, when paired with ions from NAG SAG, they usually become soluble (especially when paired with any substance from group 1, the ammonium ion, or nitrate).
CroSH ↴
– Cro = Chromates
– S = Silver
– H = Hydroxides
CarST ↴
– Car = Carbonates
– ST = Sulfides with transition metals
FluPh ↴
– Flu = Fluorides
– Ph = Phosphates
