What is Stoichiometry
Stoichiometry uses mole ratios to determine information about a reaction.
Understanding the ratio between products and reactants is very important. If you are given the amount of reactants, you can find the amount of products, and vice versa.
Ex. 2A + 3B → C + 2D
Let’s say 1 mole of A is consumed in the reaction. We can determine the amount of products formed. The ratio between A and the products is (2C / A) and (1D / A).
We can then use factor label ↴
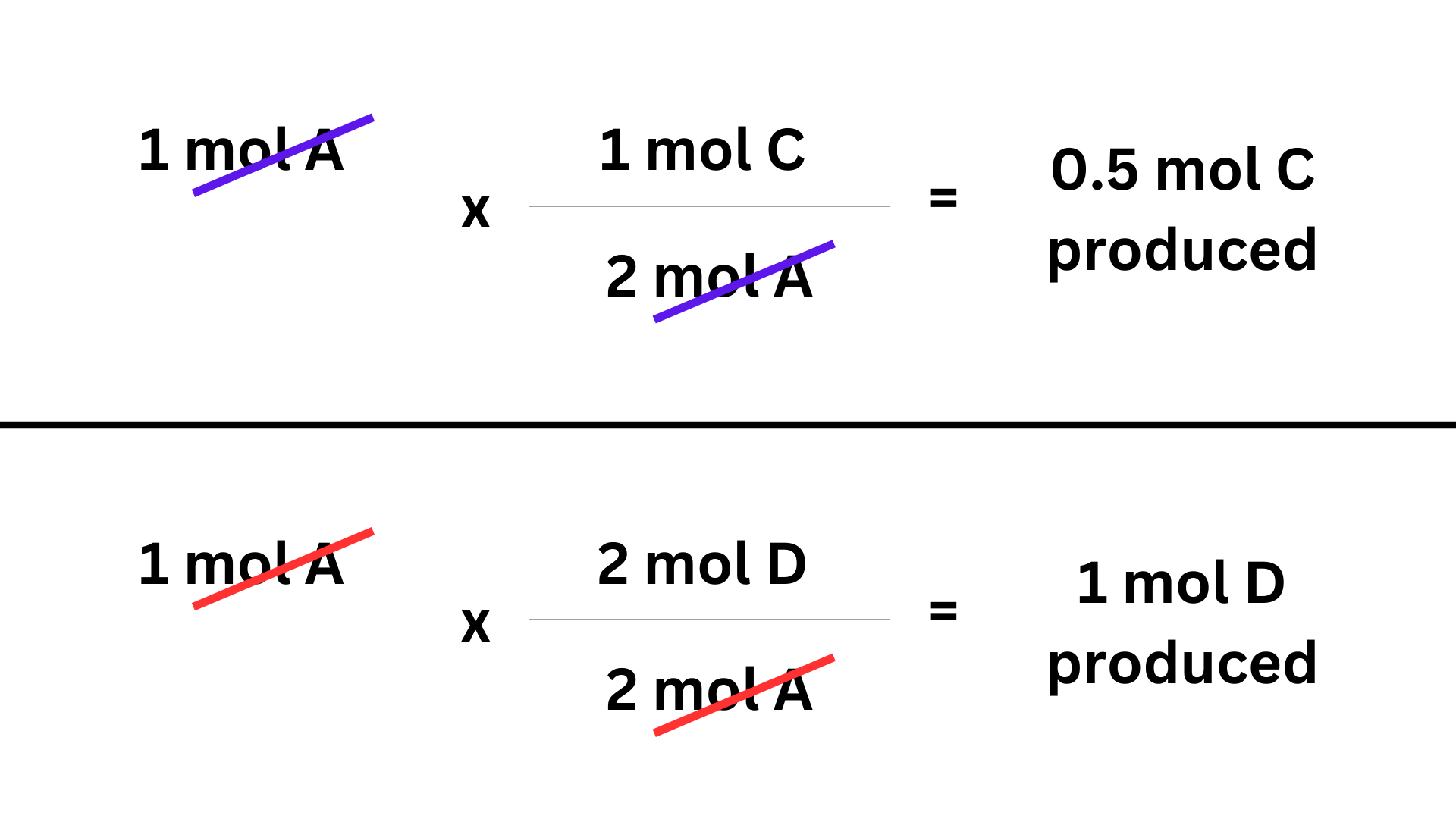
Overall, stoichiometry is a simple concept that typically is involved in more complicated problems. Do lots of practice and understand it very well, because you will need to use it throughout the year.
Limiting Reactant
The limiting reactant is the reactant that is fully consumed in the reaction. It is “limiting” because it determines when the reaction is complete.
For the reaction to occur, you need enough of all reactants to be present. When one reactant is fully consumed, the reaction cannot proceed. It would not matter if you had an infinite amount of the other reactants; the reaction ends when the limiting reactant runs out.
To determine the limiting reactant, you have to look at the stoichiometric ratios.
Ex. 2H₂ + O₂ → 2H₂O. You have 2 moles of O₂ and 3 moles of H₂

Notice how even though there are less moles of O₂, it is not the limiting reactant because of the stoichiometric ratio between the reactants.
Also note that it would not matter if we added more oxygen. Even if we have 1000 moles of O₂, only 1.5 mol reactions can occur, because all of the H₂ will be consumed in 1.5 mol reactions.
Importance of Molar Mass
Molar mass is extremely important in stoich problems. It is the gateway between mass and moles.
The key thing to understand is that if you have moles, you can find mass, and if you have mass, you can find moles. You will almost always have a periodic table with you, which you can use to find molar mass (explained in the “Molar Mass” section). Therefore, you can always convert between moles and grams.
Often, you will be given grams, which you then need to convert to moles, and then later maybe back to grams. Using factor label and molar mass, remember that you can always convert between the two.
Example Problem
20.0 grams of AlCl₃ were consumed in the reaction below. How many grams of potassium chloride were formed?
K₃PO₄ + AlCl₃ –> 3KCl + AlPO₄
Answer

Notice how we had to convert from grams to moles, and then from moles back to grams.
