Defining the System
We’ve mentioned “the system” lots of times before, but what is “the system?” In a chemical reaction/process, the system is the object(s) that we care about. In a reaction, we may consider the compounds that are involved in the process as our system, and analyze how the system’s energy changes throughout that reaction. You get to define the system based on the important objects in a process. However, it is vital to be aware of what you are considering to be “the system.”
Here’s an analogy with money. A man goes to the bank and deposits $50 from his wallet. If your system is the wallet, the system has lost $50. If your system is the bank account, it has gained $50. If your system is both the wallet and the bank account, then no money has been gained or lost by the system.
There isn’t necessarily an incorrect way to define your system. However, certain ways can make it easier or harder to understand a process.
Defining the Surroundings
The surroundings are everything outside of the system. It can be whatever interacts with the system that we don’t “care” about or are not focused on.
In the example above, when we only consider the wallet as our system, the surroundings gain $50 (the bank account is in our surroundings because it is not in the system). However, if we consider the wallet and the bank account to be our system, the system undergoes no change, resulting in no change for the surroundings as well.
Reactions in Water
The distinction between system and surroundings is especially important when considering energy changes in aqueous reactions.
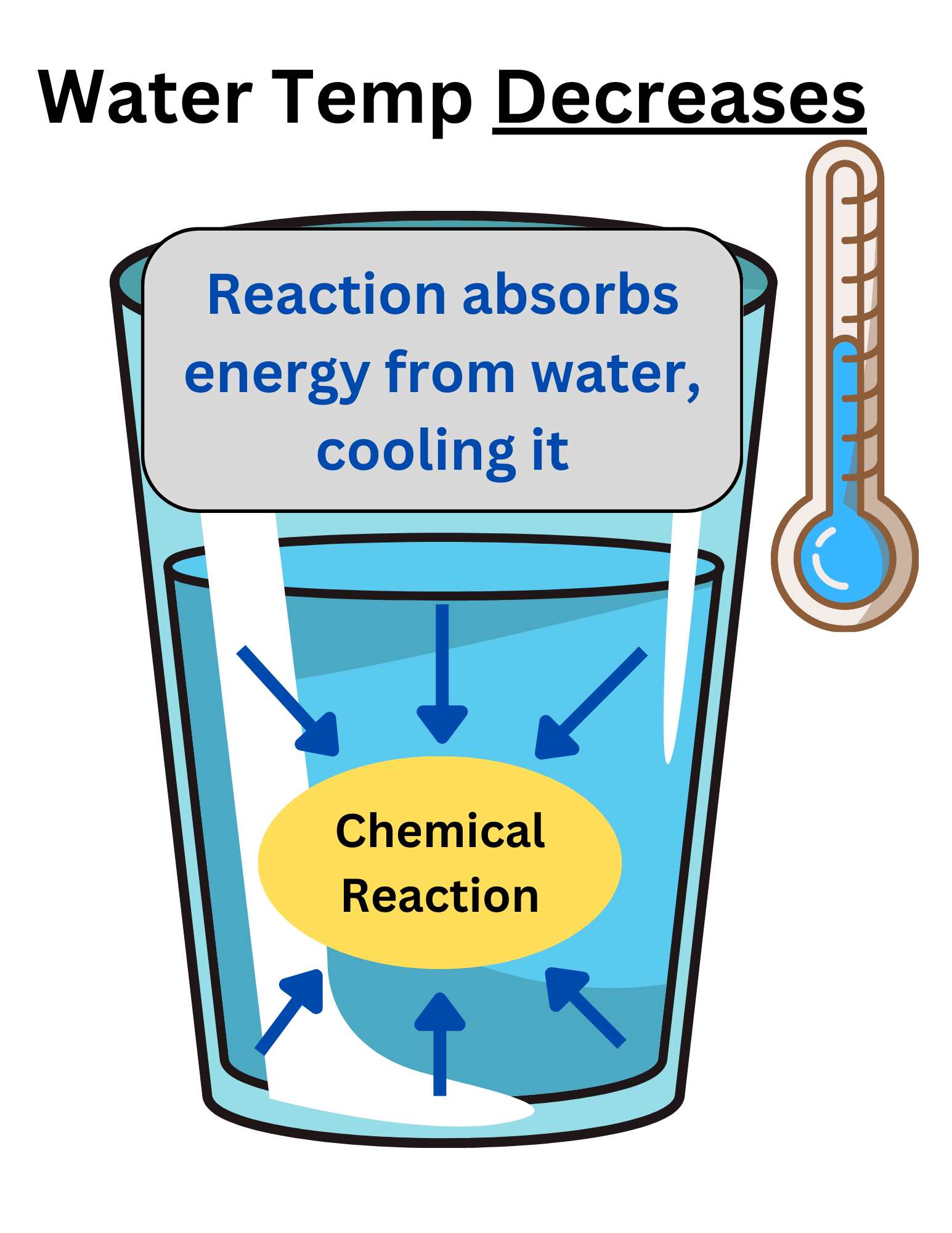
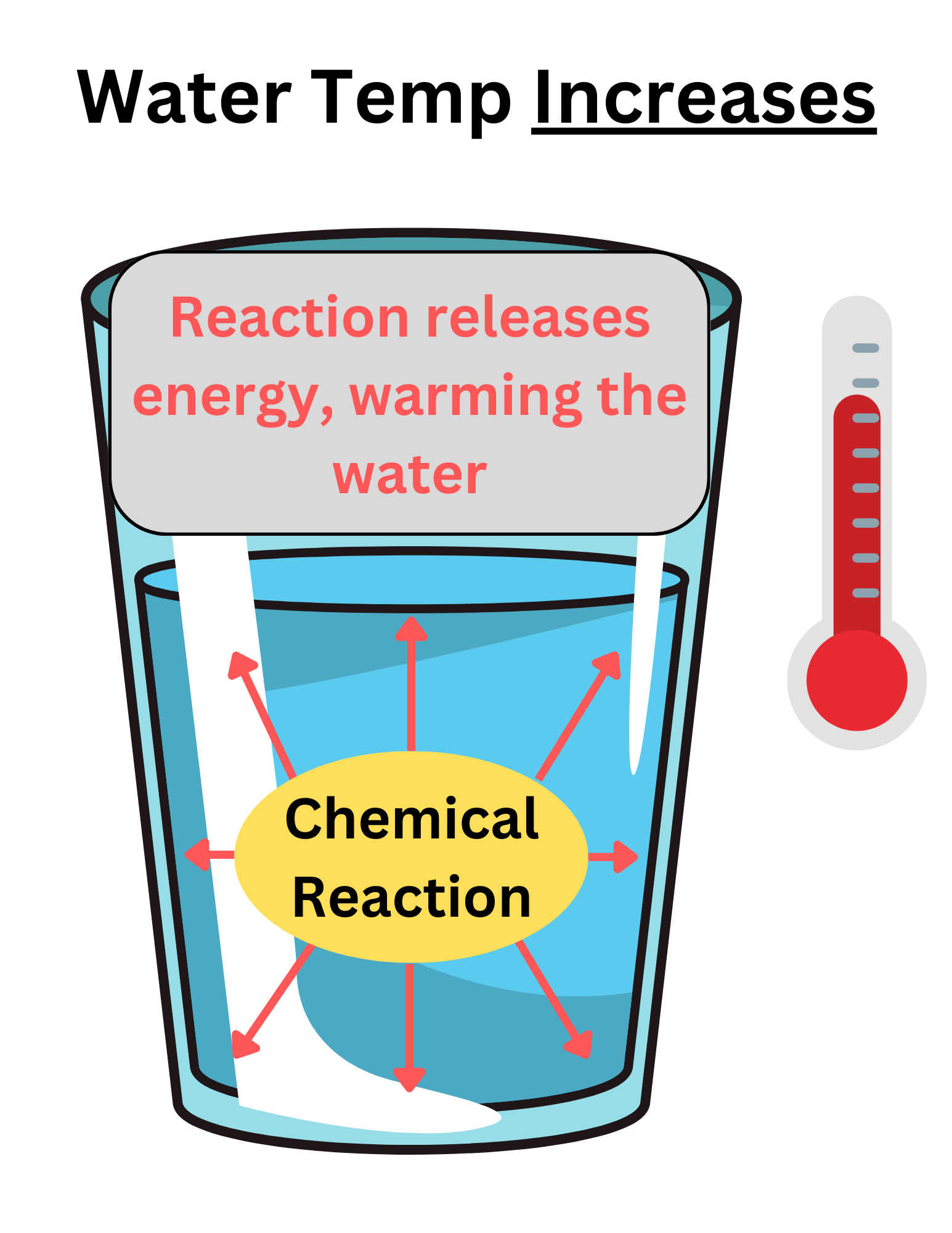
When a reaction takes place in water, if the water temp increases, the reaction is exothermic.
Many students get confused and think the reaction is endothermic because temperature increased. This is where understanding the system is important.
The water is the surroundings. It is not part of the reaction. Energy was released by the system (the reactants) into the surroundings (the water). Because the system loses/releases energy, it is exothermic.
The opposite is true when the water decreases in temperature. The system (the reactants) absorb energy from the surroundings (the water), decreasing the water temperature. Since the system gains energy, the process is endothermic.