Group: A column of the periodic table.
Period: A row of the periodic table.
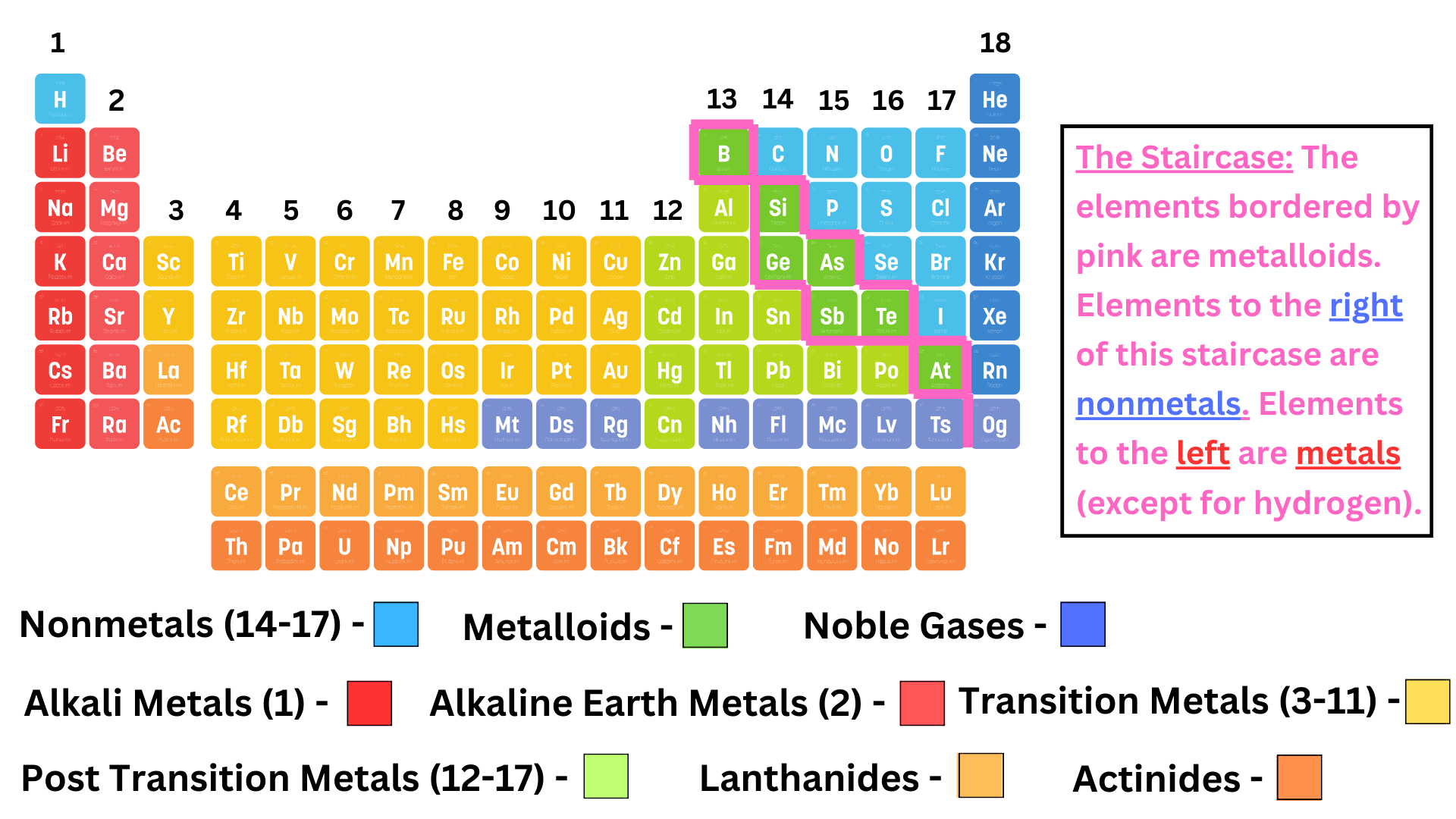
The key thing to understand is which elements are metals, and which are nonmetals. As the image above shows, there is a staircase of metalloids (which share properties of metals and nonmetals). To the left of the “staircase”, elements are metals. To the right of the “staircase”, elements are nonmetals. Metals are normally better conductors of heat and electricity than nonmetals.
Within the categories of metals and nonmetals, there are subcategories ↴
Alkali Metals: Group 1 (furthest column to the left). These metals are highly reactive with water.
Alkaline Earth Metals: Group 2, metals.
Transition Metals: In the middle of the periodic table. These metals have colored ions, and are special because they can exhibit a range of different charges (you’ll learn more about this later).
Halogens: Group 17, nonmetals.
Noble Gases: Group 18 (furthest column to the right), nonmetals. These gasses are the most stable elements on the periodic table, so they typically do not bond with other elements.
Lanthanides: At the bottom of the periodic table, you see two rows. Lanthanides are the top row. They are radioactive, and you won’t see them much throughout the year.
Actinides: Elements made through nuclear reactions. They are at the bottom of the periodic table and include elements like uranium (U), plutonium (Po), and more. You won’t see these much throughout the year.
Element Information
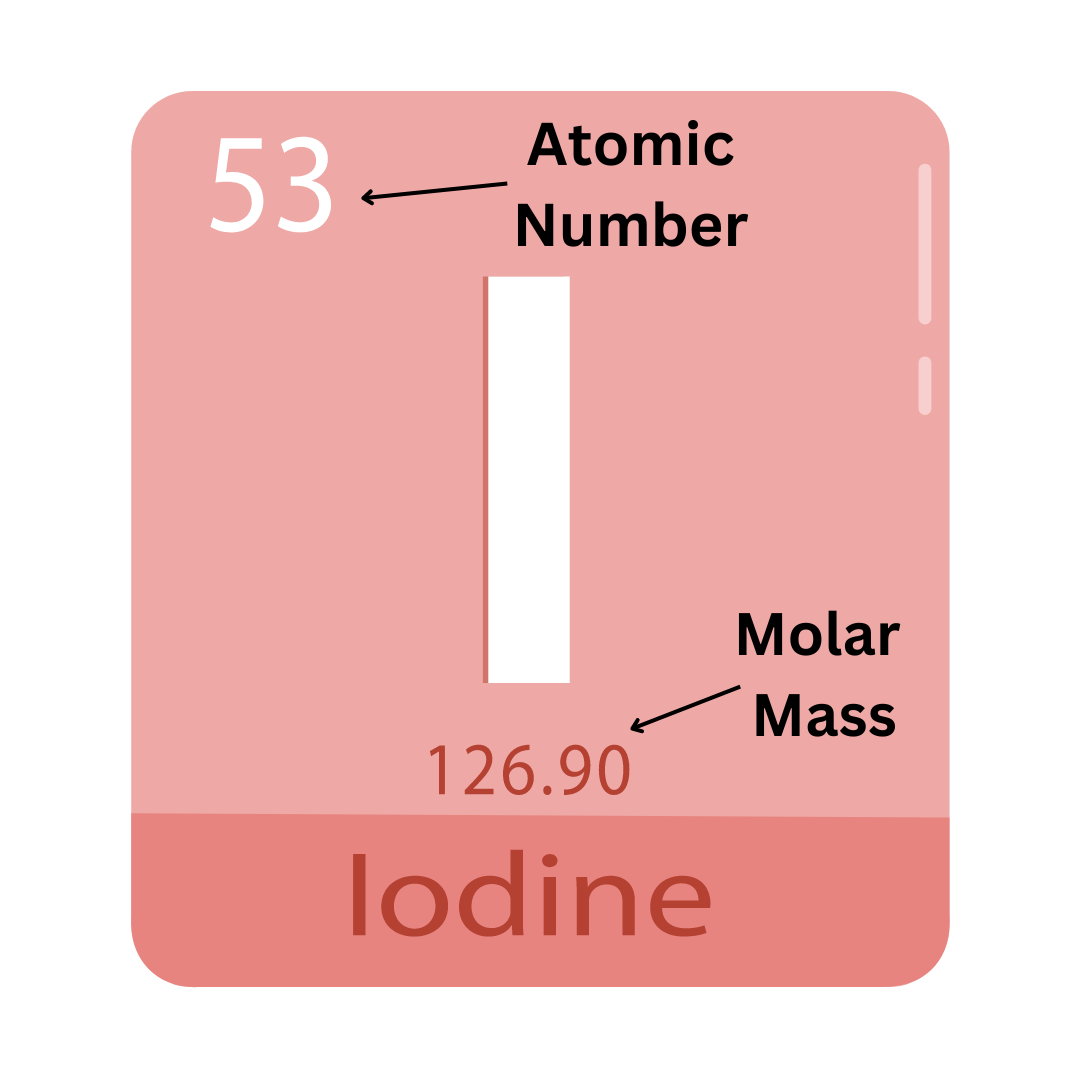
Atomic Number: Indicates the number of protons an atom has.
Atomic Mass: The actual weight of an atom in atomic mass units (amu). It is similar to the mass number (explained below), but a more exact value. Atoms of the same element sometimes have different masses, so the atomic mass you see on the periodic table is an average of those masses (you learn more about this in the “isotopes” section).
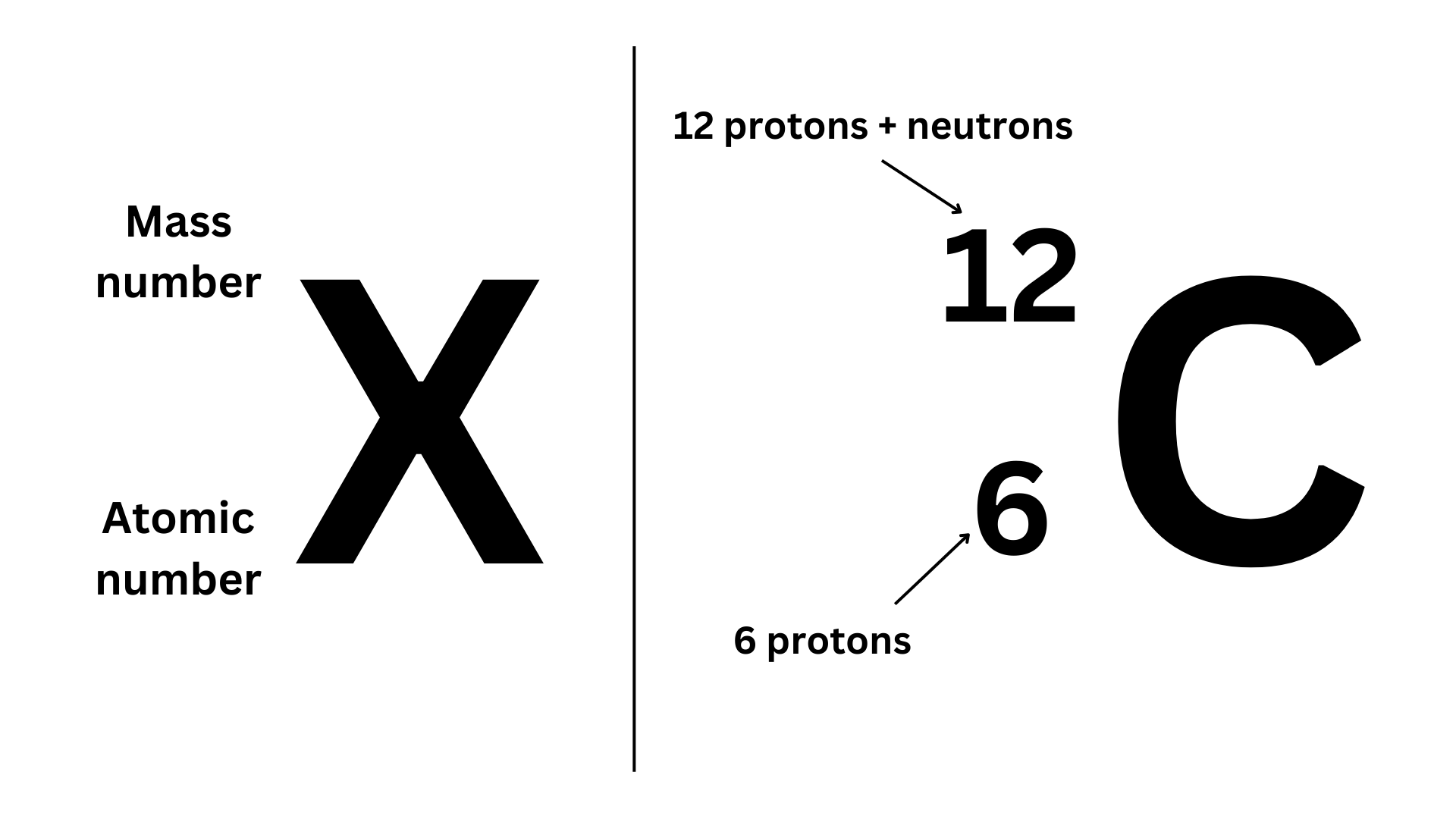
Mass Number: Indicates the amount of protons and neutrons in an atom. This can help identify isotopes of an element (you will learn about isotopes later).
Isoelectronic: When two different substances have the same number of electrons.
Ex. Mg²⁺ and Ne both have 10 electrons. The Mg atom has twelve, but because it has a 2+ charge, it has lost 2 electrons (so now it has 10). Ne already has 10 electrons, so they are are isoelectronic.
