As we discussed in the “Real vs. Ideal Gasses” section, real gasses do not perfectly follow the ideal gas law (PV = nRT). The Van der Waals equation is a modified version of the ideal gas law that takes into account the real volume of the gas particles and the interparticle attractions between gas molecules. Both of these are factors treated as negligible in the kinetic molecular theory and in PV = nRT. This is okay when dealing with ideal gasses, but the Van der Waals equation makes adjustments that help us better look at real gasses ↴

As you can see, there are two common forms of the equation. They are the exact same mathematically, just rearranged in different ways.
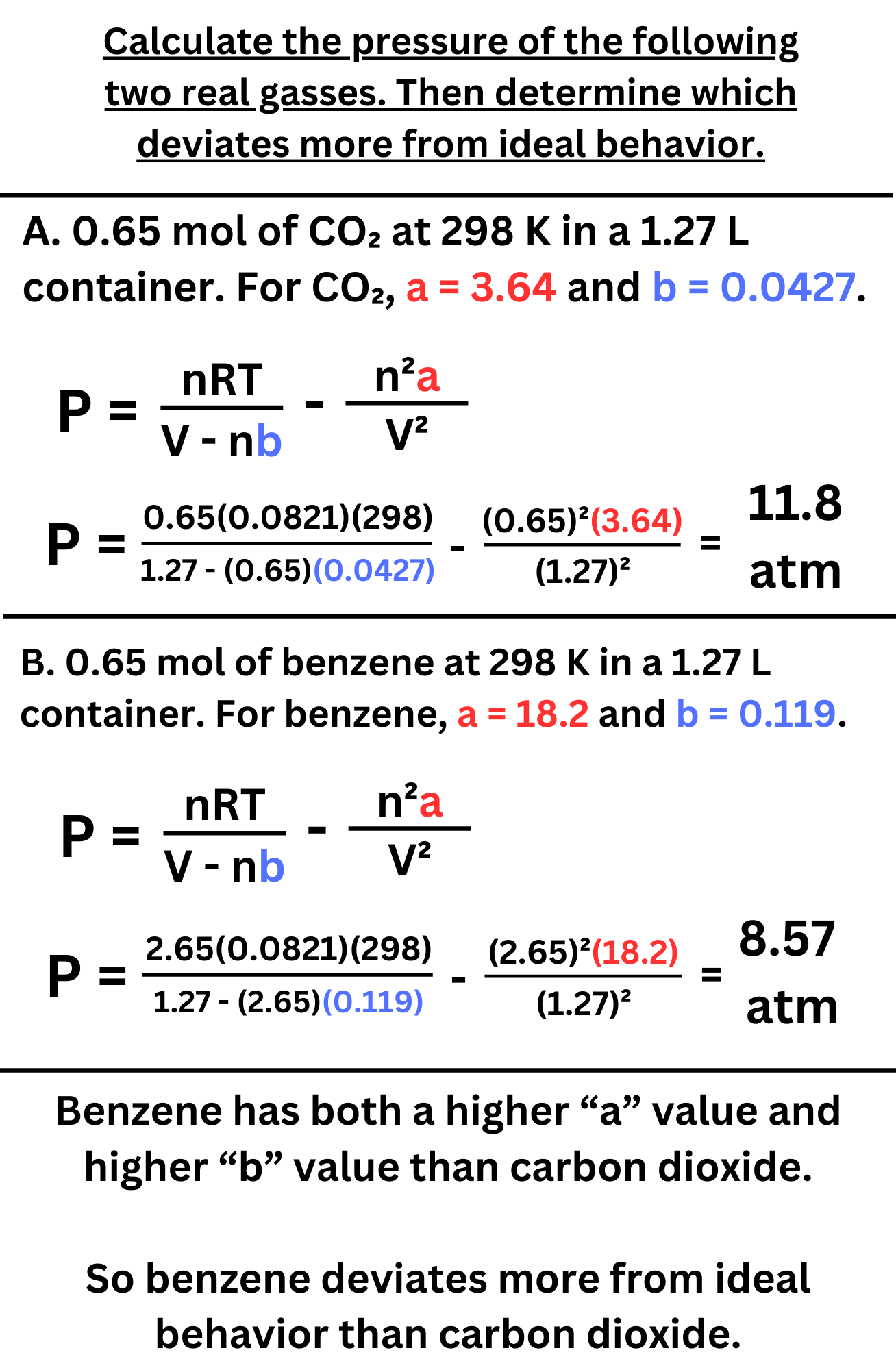
This equation modifies PV = nRT by adding two constants, a and b. a is a constant that takes into account the intermolecular forces of a gas. b is a constant that takes into account the real volume of a gas. The value of these constants is different from gas to gas. Gasses with greater a and b values deviate more from ideal behavior.
Example Problem
Calculate the pressure of the following two real gasses. Then determine which deviates more from ideal behavior.
Answer