What is Wave-Particle Duality
This is a concept not even fully understood by scientists, so don’t feel like you need to know why it happens, but just understand that it happens.
Essentially, some particles, like light, can act as both waves and particles. When we observe them, or measure their position, they behave as particles. When we don’t make measurements, they move and behave like waves.
Again, this topic is not fully understood, but just know that if something experiences wave particle duality, it sometimes behaves like a wave and sometimes like a particle.
Light
Light experiences wave-particle duality. We called individual packets/particles of light photons. Photons are discrete packets of energy with no mass.
Photons act as particles when we measure their position, or when they collide with other things.
However, we also commonly think of light as being a wave. When light is being emitted, it exhibits wave behavior. Thus, light experiences wave-particle duality.
Electrons
Electrons also experience wave-particle duality. This was proved through Young’s Double Slit Experiment. At first, the experiment was carried out using light.

This diagram is an overhead view of the experiment when the path of the light was not observed. Photons, behaving as waves, would pass through both slits, splitting into two waves. These waves would interfere with each other, creating an interference pattern on the screen. As you can see in the diagram, some areas of the screen were light, and some were dark. The only explanation for this interference pattern is that the light was behaving as a wave.
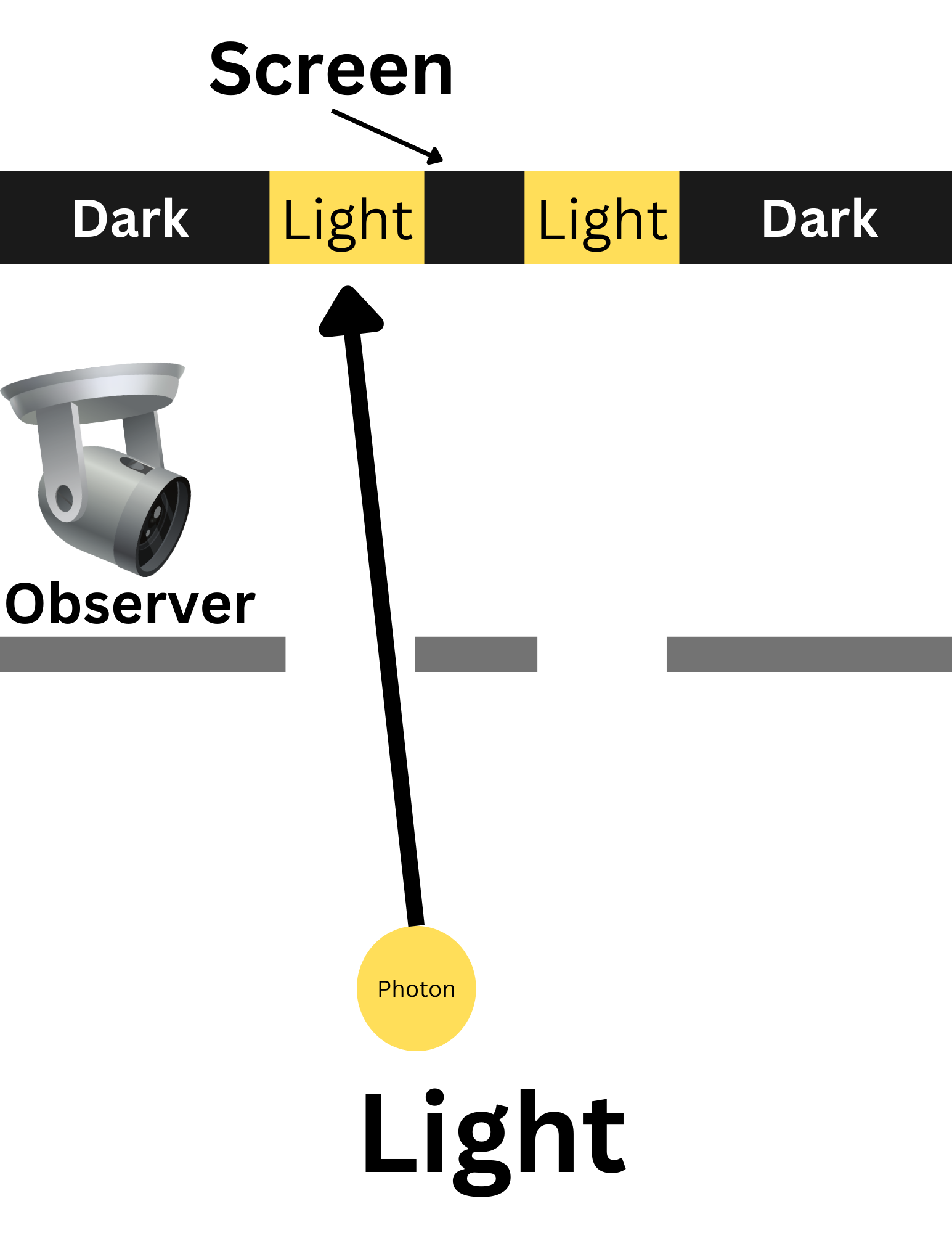
This diagram is an overhead view of the experiment when the path of the light was observed. They measured which slit each photon went through. In this experiment, the photons acted as particles, so they only went through one slit. When not observed, they passed through both slits, acting as waves. Because they are acting as particles, the photons do not interfere with each other, and there is no interference pattern. Instead, there are just two strips of light aligned with the slits.
De Broglie Equation
The De Broglie equation relates a substance’s wave behavior to its particle behavior through wavelength and momentum.
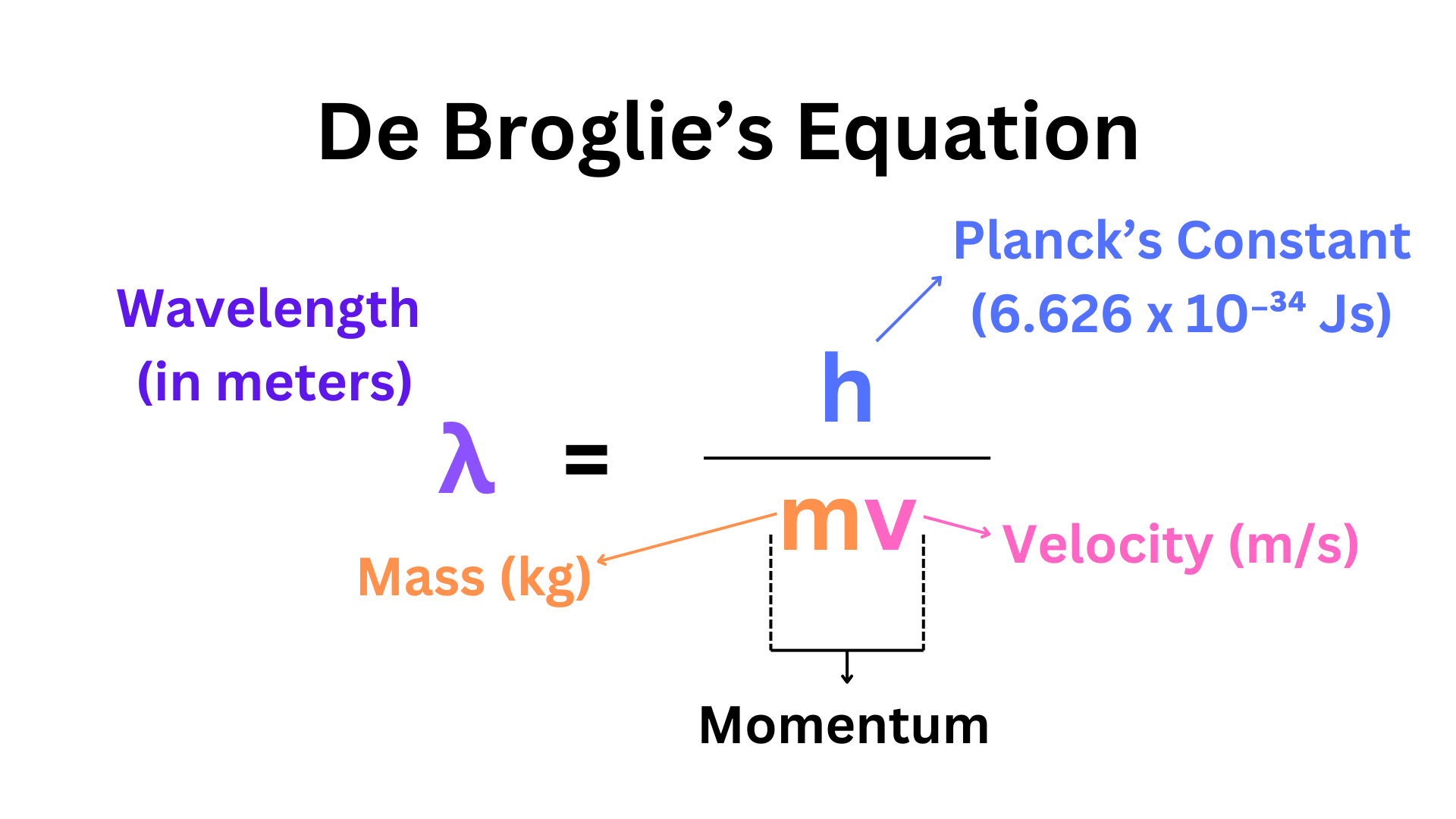
This equation can be used to calculate the wavelength of anything. You could even calculate your own wavelength (it would be very small). However, typically in chemistry, we use it to measure the wavelength of electrons.
Heisenberg Uncertainty Principle
Δx * Δp > h/(4𝝿)
– Δx = uncertainty in position
– Δp = uncertainty in momentum
– Δx and Δp are inverses
You won’t need to actually use this equation, but it’s important to understand. Because, Δx and Δp are inversely proportional, as one decreases, the other must increase. Therefore as we know more about a particle’s position, we know less about its momentum, and vice versa. This principle says that we can never exactly know both.
